Influence of PEG Content on FT-IR Absorption Signals in Silica-Based Materials Synthesized via Sol–Gel Method
Abstract
The sol–gel route is a chemical technique useful for synthesizing hybrid materials applicable to several fields such as biomedical. Through the sol–gel method, it is possible to tailor the size and control the porosity, as well as, to obtain pure hybrid materials. In this study, several inorganic–organic silica/PEG (polyethylene glycol) hybrids have been synthesized through the sol–gel method, by using tetraethylortosilicate (TEOS) as the SiO2 precursor. The PEG amount was 6, 12, 24, and 50 wt.% to SiO2 stoichiometrically calculated. The inorganic phase is well-known in literature as bioactive, while the organic phase improves mechanical performance. The influence of the organic phase has been investigated through the Fourier-transform infrared spectroscopy (FT-IR) in the range of 4000–400 cm−1. A deconvolution study of the main Si─O─Si stretching vibration in the range 1600–800 cm−1 has also been carried out. According to the observation of FT-IR spectra, the changes in the shape of O─H stretching and banding, as well as the presence of CH2 and CH3 vibrations in silica/PEG spectra revealed the co-presence of both phases. This finding is strengthened by the presence of C─O bending found in the deconvoluted spectra affecting the intensity and the shape of the Si─O─Si vibration band.
1 Introduction
Silica-based hybrid materials are a unique class of biomaterials that integrate an inorganic matrix, typically composed of a SiO₂ structure (silica), with an organic component.[1] This combination leverages the advantageous properties of both inorganic and organic phases, resulting in materials[2] with enhanced biological and mechanical characteristics.[3] These materials, referred to as biphasic, consist of organic and inorganic components mixed at the nanoscale. The unique properties of these hybrids stem from the synergistic interactions between the two phases, rather than simply combining the individual characteristics of each. Organic–inorganic hybrid materials are broadly classified into two types. In class I hybrids, the organic and inorganic components interact mainly through weak forces like hydrogen bonding and van der Waals interactions. Conversely, class II hybrids are characterized by strong chemical bonds, such as covalent or ionic-covalent bonds, that link the two phases together.[4] The scientific literature reports several studies on silica-based hybrid materials incorporating various organic molecules, investigating their chemical structure and the implications for their biological properties.[5, 6] A valuable method to produce organic–inorganic hybrid materials from solutions containing metal or metalloid precursors is the sol–gel method. This technique provides many advantages, including precise control over material properties such as porosity and composition, all at relatively low-processing temperatures.[7] The organic component, polyethylene glycol (PEG), adds beneficial properties to the hybrid material improving mechanical properties,[8] processability, and surface interactions with biological systems. Class I silica-based hybrid materials were synthesized by incorporating PEG at various concentrations (6%, 12%, 24%, and 50% wt.) to investigate the impact of this organic polymer on the silica matrix structure. The effects were analyzed using FTIR spectroscopy, with a particular focus on the Si─O bond, studied through deconvolution analysis.
2 Results and Discussion
2.1 FTIR Analysis
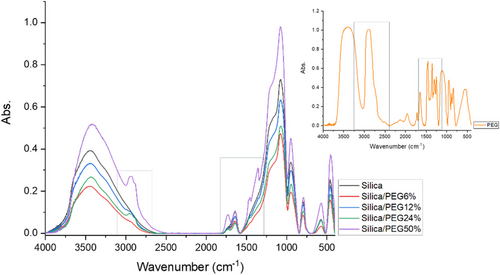
The inorganic–organic hybrids obtained by sol–gel was analyzed using FT-IR spectroscopy (Figure 1). The spectra for silica, silica/PEG hybrids and the PEG (used as reference) showed the influence of PEG concentration on the hybrids. In fact, an increase in polyethylene glycol amount provided a broadening effect on -OH stretching bond in the 3700–3300 cm⁻¹ and a rise of the CH₂ and CH₃ stretching vibrations (3000–2850 cm⁻¹).[9] Additionally, all hybrid materials retain the key peaks of the silica spectrum as the Si─O─Si absorption band at 1080 cm⁻¹ (with a characteristic shoulder at 1200 cm⁻¹) and the Si─O bending vibration at 460 cm⁻¹.[10]
2.2 FT-IR Deconvolution
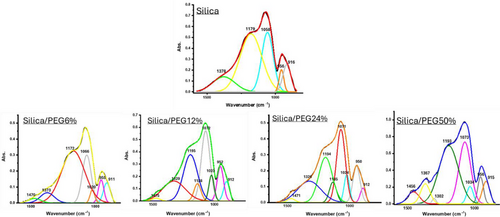
A deconvolution analysis of the Si─O band in the range of 1600–750 cm⁻¹ provides a clearer understanding of the impact of PEG on the silica matrix and the overall structure of the organic–inorganic hybrid materials (Figure 2). The asymmetric and symmetric stretching signals of Si─O─Si are evident in the peaks at 1058 cm⁻¹, attributed to the transverse optical (TO) mode, and 1179 cm⁻¹, attributed to the longitudinal optical (LO) mode. The bands at 956 and 916 cm⁻¹ correspond to Si─O and Si─OH vibrations from surface silanol groups.[11] The presence of residual ethanol in the silica matrix was indicated by the band at 1378 cm⁻1. It can be observed that the asymmetric and symmetric stretching vibrations of Si─O shift to higher wavenumbers, while the bending vibrations of Si─O and Si─OH do not exhibit any significant changes following the addition of PEG.[12] The addition of 6% PEG provided the appearance of a new band at 1470 and 1020 cm⁻¹ corresponding to the C─H vibration and C─O stretching vibration, respectively.[13] Furthermore, the C-O-H stretching mode of PEG, typically found near 1060 cm⁻¹, overlaps with the main Si─O─Si absorption band. As the PEG content increases, this peak shifts to higher wavenumbers (from 1020 cm⁻¹ in the Silica/PEG6% system to 1034 cm⁻¹ in the silica/PEG50% system), suggesting an increase in H-bonding interactions involved between the inorganic and organic phases. new peaks at 1135–1138 cm⁻¹ were observed in hybrid systems with the highest content of PEG attributed to C─O stretching contribution.[14] At 50% PEG, this peak merges with the Si─O─Si asymmetric stretching band, indicating its equivalence to the C─O vibration. The band at 1302 cm⁻¹ in the Silica/PEG50% system is attributed to the O─H bending of ethanol residue within the system.[15]
3 Conclusion
In this work, the silica/PEG hybrid systems, synthesized via the sol–gel route, have been deeply characterized thought the use of FT-IR spectroscopy. The co-presence of both the inorganic and organic phases was confirmed was strengthened by the presence of both silica and silica/PEG hybrids suggesting the hybrid formation. Moreover, an increase in PEG amount led to an increase in PEG signals in the hybrids systems. The deconvolution spectra in the range of 1600–750 cm−1 clearly showed that the main Si─O─Si absorption band of Silica is affected by C─O absorption band of PEG. The slight shifts in these signals could suggest that both the phases are interacting together. The effective interaction between the PEG polymer and the silica matrix enhances the processability of the synthesized material, while also improving its biocompatibility with human tissues for biomedical applications. This increased biocompatibility helps to reduce side effects associated with inflammatory reactions following implantation.
4 Experimental Section
Synthesis via Sol–Gel Route
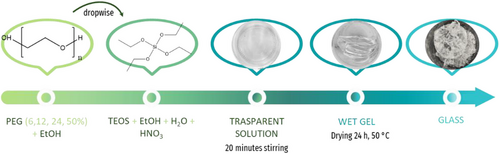
The sol–gel route was employed to synthesize a hybrid material consisting of silica and polyethylene glycol (PEG). Tetraethyl orthosilicate (TEOS) from Sigma–Aldrich was added to pure ethanol under continuous stirring to serve as the precursor for the silica inorganic matrix. Distilled water was subsequently introduced, followed by the addition of nitric acid (HNO3) to the solution. A solution of polyethylene glycol 400 (PEG) was prepared in ethanol, achieving concentrations of 6, 12, 24, and 50 wt.% relative to the amount of SiO2. The molar ratios used in this synthesis were TEOS:EtOH:H₂O:HNO₃ = 1:6:2:0.6. The mixture was stirred for 20 minutes and then allowed to rest until a wet gel was formed.[16] The wet gels obtained were subsequently dried at 50 °C for 24 h to obtain transparent glasses (Figure 3)
FT-IR (Fourier Transform Infra-Red Spectroscopy)
FT-IR measurements have been performed by a Shimadzu 21 system, following the KBr method as reported in Guadagno et al., 2023.[17] Briefly, 2 mg of each powder was weighted and added to 198 mg of KBr in order to reach a 200 mg disk for the analysis. FT-IR analysis was performed using the Prestige21 Shimadzu system (Shimadzu Italia S.R.L., Milan, Italy), equipped with a DTGS KBr detector (Shimadzu Italia S.R.L., Milan,
Italy) was employed for FT-IR analysis. Spectra were acquired with a resolution of 2 cm−1 and 60 scans in a range of 400–4000 cm−1. The software packages IR Solution and Origin were used to process the recorded spectra. The Multiple Peak Fit tools in Origin 8 software was used to deconvolute the main absorption band in the 1600–750 cm−1 range.
Acknowledgements
The authors have nothing to report.
Open access publishing facilitated by Universita degli Studi della Campania Luigi Vanvitelli, as part of the Wiley - CRUI-CARE agreement.
Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
Antonio D'Angelo, Marialuigia Raimondo, Liberata Guadagno, and Marika Fiorentino: All authors contributed equally to the entire work.
Open Research
Data Availability Statement
The data presented in this study are available on request from corresponding author.




