Silica-Based Hybrids Incorporating a Natural Antioxidant for Enhanced Tissue Regeneration
Abstract
A promising strategy for obtaining a hybrid material that entraps a natural antioxidant is the sol–gel synthesis method to be applied in the healthcare sector. A SiO2 matrix incorporating the flavonoid quercetin is synthesized using a hydroalcoholic environment using either nitric acid as a catalyst or achieving the same synthesis without acid. The hybrid materials are analyzed by Fourier transform infrared (FTIR) spectroscopy to investigate the structure of the inorganic matrix and the interaction with the natural compound. The synthesis induces alterations in the structure of quercetin, both in the presence of acid and in an acid-free environment, with potential implications for quercetin beneficial activity. A bioactivity test, following the protocol established by Kokubo et al. (Biomaterials, vol. 27, p. 2907, 2006) was conducted on hybrid materials. The results, analyzed via FTIR and EDS/SEM, confirmed the materials ability to induce hydroxyapatite formation on the silica surface. These findings suggest the potential application of these hybrid materials in tissue regeneration, leveraging the antioxidant, and immunoprotected properties of the incorporated molecule to mitigate postimplantation complications.
1 Introduction
In the field of tissue regeneration at implantation sites, there is substantial interest in silica-based inorganic–organic biomaterials adept at incorporating flavonoids.[1] Indeed, these hybrid systems can stabilize a molecule[2] with beneficial properties, thereby avoiding issues related to its low solubility and stability in the extracellular environment.[3] The effective incorporation of a bioactive molecule can be achieved through sol–gel synthesis, which is conducted at room temperature and avoids thermal degradation. Within the context of sol–gel synthesis, the catalyst assumes a critical role and can manifest as acids or bases, expediting hydrolysis and polymerization reactions. In our study, we synthesized silica-based hybrid materials incorporating 5% quercetin, a flavonol renowned for its beneficial role in human health. The hybrids were synthesized using nitric acid as a catalyst, and we obtained the same system without the acid, which might alter the structure of the encapsulated metabolite. Structural analysis of the materials and the incorporated quercetin was conducted using FTIR and UV–visible spectroscopy, while bioactivity was assessed to analyze the capacity of these hybrids to form hydroxyapatite on their surface.
2 Results and Discussion
2.1 Characterization of Hybrid Materials
A structural analysis of silica hybrids with quercetin (Qr) incorporated was conducted using FTIR spectroscopy. Table 1 compares the FTIR results of hybrid silica material with Qr synthesized with and without an acid catalyst. Our previous paper highlighted that silica/quercetin hybrids synthesized with nitric acid influence the quercetin structure,[4] leading to its oxidation[3] referred as QrOx. To preserve the structure of pure quercetin and avoid oxidation, we synthesized the same hybrid system without acid. In the spectrum of S, S+HNO₃, and the corresponding hybrids with Qr, the bands at 796–794 cm⁻¹ and 460–457 cm⁻¹ correspond to the bending vibrations of the Si–O–Si structure, while the peak at 1078–1087 cm⁻¹ and the shoulder at 1210–1196 cm⁻¹ correspond to the asymmetric and symmetric stretching of the Si─O─Si bond.[5] The use of HNO₃ is evident in the sharp peak at 1384 cm⁻¹, attributable to the N═O stretching.[6] The absence of acid in S shifts the shoulder at 1200 cm⁻¹ to 1196 cm⁻¹ and 1078 to 1087 cm⁻¹, indicating a less advanced polycondensation process in S. Furthermore, the peaks at 1483, 1447, and 1395 cm⁻¹, representing the O─CH/C─CH bending of reagent residues, indicate incomplete hydrolysis of TEOS. The incorporation of Qr within the silicon matrix is highlighted by the peak at 1165 cm⁻¹, attributed to the stretching of the ketone group C─CO─C, and the appearance of the peak at 1757 cm⁻¹ (SQr+HNO₃) and 1740 cm⁻¹ (SQr5), due to the C═O group vibration of the flavonoid in a hydroxybenzofuranone conformation. This confirms an oxidative process that alters the structure of Qr differently for the two organic-inorganic hybrids. The OH band was evident at 3600–3100 cm⁻¹, increasing in its broadness in SQr5+HNO₃ and SQr5, underlining the formation of new H-bonds.[7]
| Assignments | Wavenumber (cm−1) | |||||
|---|---|---|---|---|---|---|
| S+HNO3 | S | SQr5+HNO3 | SQr5 | QrOx | Qr | |
| O─H Stretching | 3700–3050 | 3650–3050 | 3750–3030 | 3750–3040 | 3600–3100 | 3500–3100 |
| C═O Stretching | – | – | 1757 | 1740 | 1757 | 1660 |
| O─CH/C─CH Bending | – | 1483, 1447, and 1395 | – | – | – | – |
| N═O Stretching | 1384 | – | 1384 | – | – | – |
| Si─O─Si Sym stretching | 1210 | 1196 | 1210 | 1210 | – | – |
| C─C(═O)─C | – | – | 1165 | 1165 | 1164 | 1165 |
| Si─O─Si As stretching | 1078 | 1087 | 1074 | 1070 | – | – |
| Si─OH | 947 | 958 | 947 | 949 | – | – |
| Si─O─Si Bending | 796 | 794 | 794 | 794 | – | – |
| Si─O─Si Bending | 460 | 463 | 457 | 461 | – | – |
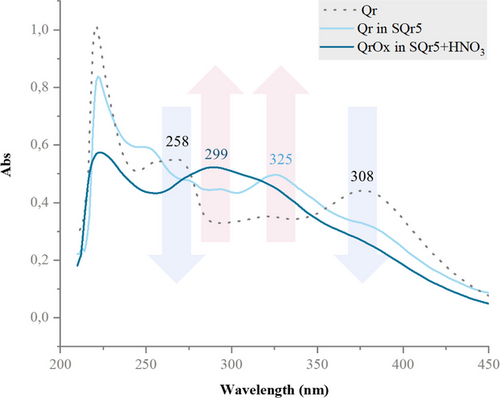
Quercetin incorporated into a silica matrix with nitric acid undergoes oxidation in aqueous and acidic conditions, altering its structure and biological activity. In pure quercetin (Qr), the UV–visible spectrum displays two main bands: 250–260 nm (band II) for the cinnamoyl group and 380 nm (band I) for the B─C ring conjugated system. In SQr5+HNO₃, these bands disappeared and are replaced by a new peak at 299 nm, indicating a rearrangement into a quinone group. For SiQ5, the slightly acidic pH due to TEOS and the hydroalcoholic environment cause a decrease in the 380 nm band and an increase at 325 nm, suggesting the formation of quercetin o-quinoids (Figure 1).
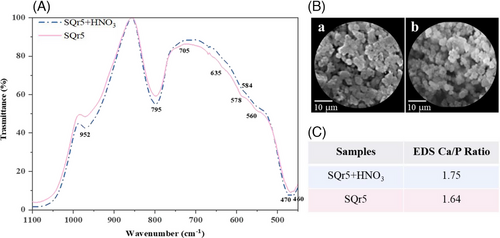
The bioactivity test evaluated hydroxyapatite (HA) formation on hybrid materials soaked in simulated body fluid (SBF) for 21 days, essential for osteointegration (Figure 2). FTIR analysis (A) revealed phosphate groups (at 635, 560, and 470 cm⁻¹). SEM showed globular HA structures (B), while EDS (C) indicated Ca/P ratios of 1.75 and 1.64 for SQr5+HNO₃ and SQr5, respectively, confirming HA formation.
3 Conclusion
The goal of this work was to create a biomaterial using environmentally friendly methods while preserving the desired properties. Our results indicate that a hybrid system can be synthesized without the use of nitric acid, as evidenced by FTIR analysis. Nevertheless, structural modifications in the encapsulated drug were observed in both approaches. In the presence of nitric acid, quercetin was oxidized into 3(2H)-benzofuranone, whereas, in its absence, the oxidation in the hydroalcoholic medium resulted in the formation of a six-membered lactone ring. Both hybrid materials successfully facilitated the formation of hydroxyapatite, as confirmed by FTIR and SEM/EDS analyses. This continuing research is directed towards the development of innovative biomaterials with superior performance.
4 Experimental Section
Synthesis via Sol–Gel Route
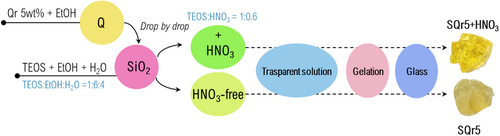
The sol–gel method was used to synthesize a silica structure with 5% quercetin (Qr) under two conditions. Tetraethyl orthosilicate (TEOS) from Sigma–Aldrich was dissolved in ethanol and distilled water with magnetic stirring. In the first synthesis, quercetin, dissolved in ethanol, was added to the silica solution, followed by nitric acid to accelerate hydrolysis and polycondensation, resulting in the hybrid system SQr5+HNO3. In the second synthesis, no acid was added, producing a different hybrid system, SQr5. After gelation, the samples were dried at 50 °C for 24 h to form a glassy material. The procedure is illustrated in Figure 3.
Characterization of Hybrid Materials
A Prestige21 Shimadzu system, equipped with a DTGS KBr detector, was employed for FTIR analysis using a range of 400–4000 cm−1 and a resolution of 2 cm−1 (60 scans). Disks were prepared using 198 mg of KBr and 2 mg of ground hybrid materials for analysis. The FTIR spectra were processed using IRsolution and Origin 8 software. Silica matrix samples without organic compounds synthesized in the presence and absence of acid, along with pure quercetin, were used as references for the hybrid systems. A T65 UV–visible spectrophotometer (PG Instruments, UK) was used to record the spectra of quercetin in hybrid systems, analyzing structural changes from sol–gel synthesis in acid and acid-free conditions. SQr5+HNO₃ and SQr5 samples were ground and quercetin was extracted via ultrasound-accelerated maceration in ethanol. The extracts spectra were measured from 200 to 600 nm, along with the spectrum of pure quercetin in ethanol for comparison.[8]
Bioactivity Test
The in vitro bioactivity study was conducted following Kokubo test.[1] Discs, each weighing 250 mg, were prepared from each sample and immersed in simulated body fluid (SBF) for 7, 14, and 21 days. SBF was prepared as reported in our previous paper,[9] and the discs were placed in SBF maintained at 37.0 ± 0.5 °C. After up to 21 days of immersion, Hydroxyapatite formation was evaluated using FTIR and scanning electron microscopy (SEM, Quanta 200, FEI, Eindhoven, The Netherlands) coupled with energy dispersive X-ray spectroscopy (EDS).
Acknowledgements
Open access publishing facilitated by Universita degli Studi della Campania Luigi Vanvitelli, as part of the Wiley - CRUI-CARE agreement.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data presented in this study are available on request from corresponding author.




