Sequence-Controlled Copolymerization of Structurally Well-Defined Multinuclear Zinc Acrylate Complexes and Styrene
Abstract
The copolymerization of two or more monomers produces polymeric materials with unique properties that cannot be achieved with homopolymers. However, precise control over the polymer sequence remains challenging because the sequence is determined by the inherent reactivity of comonomers. Therefore, only limited methods using modified monomers or supramolecular interactions are reported. In this study, the sequence control of acrylate-styrene copolymerization using multinuclear zinc complexes is reported. The copolymerization of the zinc acrylate complex with a polymeric sheet-like structure and styrene in benzene affords a copolymer with a higher content of acrylate triad than calculated for the statistical random model, whereas tetranuclear zinc acrylate (TZA) affords a copolymer with fewer adjacent acrylate sequences. The copolymer with a higher content of acrylate triad exhibits a lower glass transition temperature because of the higher mobility of the longer polystyrene segments. These results highlight the promise of multinuclear zinc acrylate complexes as monomers for sequence-controlled copolymerization.
1 Introduction
Copolymers composed of two or more monomers are commonly used in practical material applications because the properties can be easily modified by changing their compositions and other factors. Monomers typically undergo chain-growth radical copolymerization to produce copolymers with statistically random sequences of more than one monomer in the main chain of the copolymer. Since both the composition and sequence distribution of the monomers significantly impact the physical properties of copolymers, the methodology for controlling the sequence is a state-of-the-art research topic in polymer science that can achieve different polymer properties from those of random copolymers.[1-3] Sequence-controlled copolymerizations are mainly achieved by an AB-type sequence. Copolymers with the AB-type sequence altered by combining electron-rich and electron-deficient monomers.[4-16] Ouchi demonstrated that sterically bulky methacrylamide alternatively copolymerized with styrene and could be converted into methacrylic acid and alkyl methacrylate residues after copolymerization.[17, 18] The sequence-controlled radical copolymerization of two or more monomers has been achieved through various technologies. These include employing sequence-controlled trimer as a monomer,[19] small molecular template,[20, 21] supramolecular templates,[22] metal–organic framework as a template,[23] Lewis acid–base interaction,[24] reversible addition-fragmentation chain transfer agent,[25] sequential feeding of more reactive monomers,[26-28] and the formation of micelle.[29-31] However, although these methods precisely control the polymer sequence, they often require the use of modified monomers, which compromises the synthesis of these monomers in some cases.
As an alternative monomer for controlling polymer sequences, we are interested in the structural diversity of zinc acrylate. Zinc acrylate, which consists of a Zn2+ ion and two acrylate anions, has been used as a comonomer in the copolymerization with styrene.[32] However, owing to the lack of structural regularity, acrylate moieties were randomly incorporated into the main chain (Figure 1A).[33] Sherrington demonstrated the sequence-controlled copolymerization of zinc acrylate with styrene using (−)-sparteine, which is a sterically hindered diamine ligand, to form the tetrahedral coordination geometry of the Zn2+ ion, where two acrylate moieties on the Zn2+ ion are brought close together by sparteine and incorporated adjacent to the main chain (Figure 1B).[34, 35] These results confirmed that the use of mononuclear zinc acrylate enabled the incorporation of only two adjacent acrylates into the copolymer.
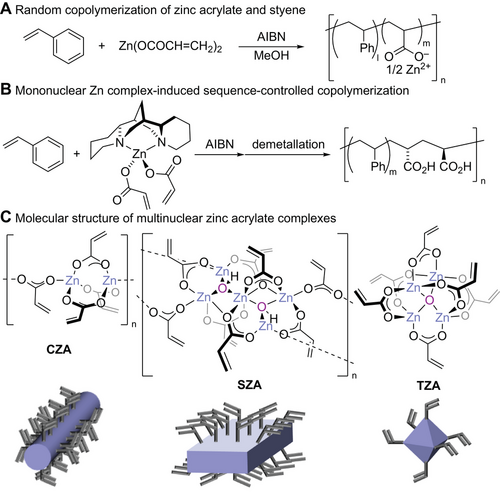
We recently reported the structural diversity of zinc acrylate complexes (Figure 1C).[36] The structure of commercially available zinc acrylate, Zn(OCOCH═CH2)2, was determined to be an infinite chain structure, [Zn2(OCOCH═CH2)3(OCOCH═CH2)]n (chained zinc acrylate, CZA), consisting of a dinuclear Zn2(OCOCH═CH2)3 fragment and cross-linking acrylate. Recrystallization of zinc acrylate from wet THF afforded crystals containing a hydroxido-bridged pentanuclear unit, Zn5(μ-OH)2(OCOCH═CH2)8, with a sheet-like structure as the repeating fragment of the infinite structure [Zn5(μ3-OH)2(OCOCH═CH2)8]n (sheet-like zinc acrylate, SZA). When zinc acrylate was treated with ZnO, a tetranuclear μ-oxo complex, Zn4(μ4-O)(OCOCH═CH2)6 (tetranuclear zinc acrylate, TZA), was obtained. In sharp contrast to the mononuclear zinc acrylate complexes used in previous studies, the multinuclear zinc complexes reported in this study possess multiple acrylate ligands on the surface of the multinuclear core and can be easily obtained from commercially available zinc acrylate. Therefore, the structural diversity and accessibility of these zinc acrylate complexes prompted us to investigate their copolymerization with styrene to control the acrylate sequence. This study provides a detailed structural analysis and reports the effect of the sequence on the physical properties of the copolymers, which have not been previously addressed.
2 Results and Discussion
We determined the solubility of zinc acrylate complexes in organic solvents (Table 1) because CZA and SZA have infinite structures in the solid state. When MeOH was used as the solvent, these complexes demonstrated a sufficient solubility of ≈50 g L⁻1, with approximately half amount of zinc acrylate complex being soluble to give a heterogeneous reaction mixture in all cases. In contrast, CZA and SZA exhibited low solubilities of 2.1 and 4.4 g L⁻1, respectively, in non-polar benzene, whereas TZA exhibited a high solubility of 49 g L⁻1. The higher solubilities of CZA and SZA in MeOH were likely caused by the replacement of cross-linked acrylates by MeOH molecules to dissociate the repeating units. In fact, molecular weight measurements of CZA in a polar solvent, namely dimethyl carbonate, using vapor pressure osmometry revealed that zinc acrylate exists as a dinuclear structure coordinated by dimethyl carbonate.[36] Conversely, the low solubilities of CZA and SZA in benzene suggested that these complexes maintained an infinite structure in non-polar benzene as in the crystal. Therefore, we chose MeOH and benzene as solvents for copolymerization with styrene to investigate the effect of the molecular structure of the zinc acrylate complexes on the sequence and physical properties of the copolymers.
 |
|||||||||
| Entry | Zn complex | Solvent | Solubility [g L−1] | Copolymer | Yield [%] | i.r. [mol%]b) | Mn (103)c) | Mw (103)c) | PDIc) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CZA | MeOH | 47 | 3CZA | 63 | 18.3 | 339 | 2057 | 6.1 |
| 2 | SZA | MeOH | 46 | 3SZA | 65 | 18.0 | 896 | 4730 | 5.3 |
| 3 | TZA | MeOH | 61 | 3TZA | 59 | 14.5 | 427 | 2316 | 5.4 |
| 4 | CZA | benzene | 2.1 | 4CZA | 20 | 2.0 | 50 | 103 | 2.1 |
| 5 | SZA | benzene | 4.4 | 4SZA | 18 | 4.0 | 48 | 99 | 2.0 |
| 6 | TZA | benzene | 49 | 4TZA | 57 | 15.3 | 126 | 549 | 4.4 |
- a) Conditions: Styrene (3.2 g), zinc acrylate complex (0.8 g), and AIBN (15–22 mg) in solvent (6 mL) at 60 °C for 19 h. Yields of copolymers 1 and 2 are given in the Table;
- b) Determined by NMR analysis;
- c) Determined by size exclusion chromatography (SEC) using polystyrene standard.
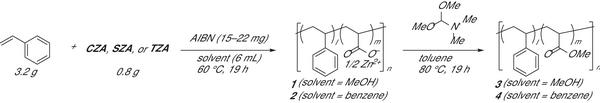
Copolymerization of zinc acrylate complexes with styrene was conducted in MeOH or benzene at 60 °C for 19 h using AIBN as a radical initiator to obtain poly(styrene-co-zinc acrylate) 1 and 2, respectively, where the reactivity ratio between zinc acrylate and styrene is known to be ≈1.[32] Copolymers 1 and 2 were dried, washed with MeOH by Soxhlet extraction, extracted with HCl/MeOH to remove Zn2+ ions, and treated with a methylating reagent, N,N-dimethylformamide dimethyl acetal, to convert them into poly(methyl acrylate-co-styrene) 3 and 4 for NMR and SEC analyses (Figures S7–S18, Supporting Information). The removal of Zn2+ ions and conversion of carboxylic acid moieties into methyl esters were confirmed by IR analyses (Figures S1–S6, Supporting Information). Table 1 summarizes the obtained copolymers. In MeOH, CZA, SZA, and TZA afforded copolymers 3CZA, 3SZA, and 3TZA, respectively, with incorporation ratios (i.r.) of 14.5–18.3 mol% and Mn values of 339000–896000 (entries 1–3). The acrylate moiety in each polymer chain was calculated to be ≈600. However, the parameters of copolymers 4 prepared in benzene largely varied depending on the zinc acrylate complex used (entries 4–6). Copolymers 4CZA and 4SZA obtained by the copolymerization of CZA and SZA, respectively, with styrene showed low incorporation ratios of 2.0 and 4.0 mol% (entries 4 and 5). In contrast, the copolymer 4TZA prepared using TZA showed a similar incorporation ratio (15.3 mol%) to that of copolymers 3TZA (entries 3 and 6). These results suggested that the solubility of zinc acrylate complexes in the reaction media largely affected the incorporation of acrylate moieties and that less soluble CZA and SZA maintained their polymeric structures in benzene. Therefore, the acrylate sequence of the multinuclear Zn complexes could be transferred to the copolymer at the liquid–solid surface. The molecular weight of copolymers 4 prepared in benzene was less than that of 3; however, the number of acrylate residues in each polymer chain was greater than 10, even for 4CZA (entry 4).
Next, we investigated the sequence of acrylate moieties in the copolymers by NMR after conversion of them into the corresponding methyl esters 3 and 4.[37] Table 2 summarizes the triads of copolymers 3 and 4 determined by 13C NMR analysis (Figures S7–S12, Supporting Information) and calculated values, which indicated that methyl acrylate moieties were randomly incorporated into the polymer chain. In the MMM triad, a methyl acrylate moiety was neighbored both sides by two methyl acrylates, and in the MMS triad, a methyl acrylate moiety was sandwiched between the methyl acrylate and styrene moieties. Copolymers 3, obtained by copolymerization in MeOH, showed a lower content of MMS triads than the calculated values and almost the same content of MMM triads as the calculated values for the random copolymer (entries 1–3). It should be noted that copolymers 3 showed almost the same triads regardless of the structure of multinuclear zinc complexes used as the comonomer probably due to the dissociation of the multinuclear structure in MeOH. In sharp contrast to the copolymers 3CZA and 3SZA, 4CZA and 4SZA, which were obtained by copolymerization in benzene, showed higher content of MMM and MMS triads than those in random copolymers (entries 4 and 5). On the other hand, 4TZA had significantly lower content of MMS triad than that of random copolymer (entry 6).
| Entry | Copolymer | COO−/chaina) | MMM [%] | MMS [%] | ||
|---|---|---|---|---|---|---|
| Calc.b) | Obs.a) | Calc.b) | Obs.a) | |||
| 1 | 3CZA | 661 | 3 | 5 | 30 | 19 |
| 2 | 3SZA | 641 | 3 | 1 | 30 | 17 |
| 3 | 3TZA | 574 | 2 | 0 | 25 | 18 |
| 4 | 4CZA | 10 | 0 | 2 | 4 | 12 |
| 5 | 4SZA | 21 | 0 | 29 | 8 | 15 |
| 6 | 4TZA | 190 | 2 | 2 | 26 | 4 |
- a) Determined by NMR analysis;
- b) Calculated as a random sequence, where ratios show triads containing at least one M, and SSS triad is not considered. MMM: m × m / 100 (%), MMS: 2 × m × s / 100 (%), where m = i.r. of methyl acrylate (%), s = 100 – m (%).
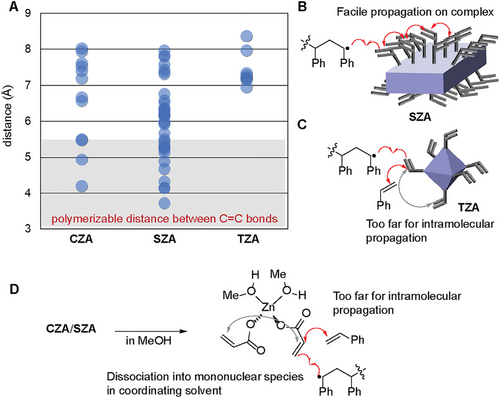
In literature, the C═C bond participated in polymerization when the distance between two adjacent C═C bonds was in the range of 5.0–5.5 Å in the solid state.[38] In a solution, the distance between two adjacent C═C bonds needed to be less than 5.4 Å for polymerization to occur.[39] The distance between the C═C bonds in TZA was considerably longer than those in CZA and SZA (Figure 2A). In addition, TZA did not have an acrylate pair that facilitated the sequential incorporation of acrylates on the molecule. Thus, both the MMM and MMS triads resulted in lower values than those calculated for random copolymers. The observed higher content of triads was likely caused by the close proximity of the acrylate moieties on CZA and SAZ in the solid state (Figure 2A) and homopolymerization of acrylate on the surface of the multinuclear complex, as shown in Figure 2B. As previously stated, the copolymerization of TZA and styrene in benzene resulted in a lower MMS value than the calculated value. This result could be explained by the isolated acrylate moieties of TZA (Figure 2C), which indicated that the densely located acrylate moieties on CZA and SZA led to a non-statistical alignment of the acrylate moieties in the copolymer and TZA enables the introduction of acrylate moieties in copolymer separately. In MeOH, the infinite structure of CZA and SZA dissociates into mononuclear zinc acrylate or oligomeric structures upon coordination of MeOH, leading to the homogeneous situation. Two acrylate moieties on the tetrahedral Zn center hardly undergo consecutive incorporation (Figure 2D). Therefore, the obtained copolymers 3CZA and 3SZA have fewer MMS triads compared to the statistical random model.
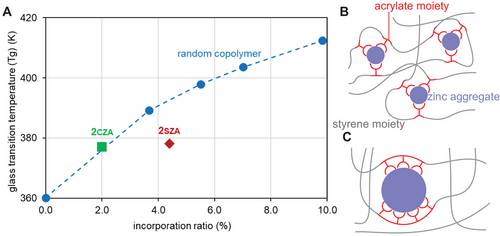
The thermal properties of non-statistically aligned poly(styrene-co-zinc acrylate) 2CZA and 2SZA were investigated. The glass transition temperature (Tg) of poly(styrene-co-zinc acrylate) increased with the incorporation ratio of the zinc acrylate moiety.[40, 41] In previous studies, random copolymers were used to investigate the thermal properties, which showed a positive correlation between the incorporation ratio of the acrylate moiety and Tg (Figure 3A, blue circles). This was because the copolymer with a higher i.r. formed more cross-linking points, which reduced the mobility of the polystyrene segments (Figure 3B). Copolymer 2CZA, with a slightly higher content of MMS triad content, showed a similar trend to the random copolymer (Figure 3A, green square), having a Tg of 377 K with a 2.0 mol% i.r. In contrast, copolymer 2SZA, with a higher content of MMM triad, exhibited a relatively lower Tg of 378 K with a 4.4 mol% i.r. (Figure 3A, red rhombus). The lower Tg of 2SZA can be explained as follows: Copolymer 2SZA had a higher content of MMM triad, which resulted in it possessing more distinctly segregated polystyrene and poly(acrylic acid) segments than the random copolymers. Segments with longer continuous acrylate sequences formed larger zinc aggregates, resulting in fewer cross-linking points in the polymer. As a result, polystyrene segments had the mobility to show a lower Tg (Figure 3C).
3 Conclusion
In summary, we investigated the copolymerization of three types of multinuclear zinc acrylate complexes with styrene. Copolymerization in non-polar benzene resulted in the non-statistical alignment of the acrylate moieties in the copolymer and especially, CZA and SZA showed higher content of MMS and MMM triads than the random copolymer while the use of TZA led to the isolation of acrylate moieties. On the other hand, the dissociation of the infinite structure of CZA and SZA in MeOH resulted in the homogeneous copolymerization between mononuclear zinc acrylate and styrene to afford a similar alignment of the acrylate moieties regardless of the solid-state structure. The non-statistical alignment of the acrylate moieties led to a change in the physical properties of the copolymers, e.g., poly(styrene-co-zinc acrylate) with a high content of MMM triad exhibited Tg of ≈10 K lower than that of a random copolymer. Because the combination of Zn2+ ions and carboxylate, hydroxido, and oxido anions exhibited high structural diversities, even without costly organic molecules as supporting ligands, this study provides a novel method to control the sequence of acrylate and related monomers in copolymerization with readily accessible multinuclear Zn complexes.
Acknowledgements
A part of this work was conducted at “Advanced Infrastructure for Materials and Nanotechnology in Japan (ARIM)” of the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Grant Number JPMXP1223UT0046. This work was partly supported by the Grant-in-Aid for Scientific Research(B) (Nos. JP23H01955 and JP24K26648) from JSPS, and the Grant-in-Aid for Transformative Research Areas (A) JP21A204 in Digitalization-driven Transformative Organic Synthesis (Digi-TOS) from MEXT, Japan (No. JP22H05340 and JP24H01061). R.M. is grateful to the Program for Leading Graduate Schools (MERIT-WINGS) and to the Grant-in-Aid for JSPS Fellows (JP23KJ0761).
Conflict of Interest
T.I. and K.N. are listed as inventors in Japan patent application on a part of this work (Japan Patent Application No. 2023-140539).
Open Research
Data Availability Statement
The data that support the findings of this study are available in the supporting information of this article.




