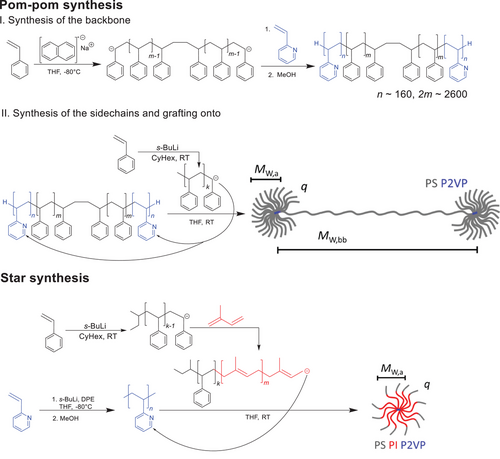
Fast and Scalable Synthetic Route to Densely Grafted, Branched Polystyrenes and Polydienes via Anionic Polymerization Utilizing P2VP as Branching Point
Abstract
Defined, branched polymer architectures with low dispersity and architectural purity are of great interest to polymer science but are challenging to synthesize. Besides star and comb, especially the pom-pom topology is of interest as it is the simplest topology with exactly two branching points. Most synthetic approaches to a pom-pom topology reported a lack of full control and variability over one of the three topological parameters, the backbone or arm molecular weight and arm number. A new, elegant, fast, and scalable synthetic route without the need for post-polymerization modification (PPM) or purification steps during the synthesis to a pom-pom and a broad variety of topologies made from styrene and dienes is reported, with potential application to barbwire, bottlebrush, miktoarm star, Janus type polymers, or multi-graft copolymers. The key is to inset short poly(2-vinyl-pyridine) blocks (<2 mol% in the branched product) into the backbone as branching points. Carb anions can react at the C6 carbon of the pyridine ring, grafting the arms onto the backbone. Since the synthetic route to polystyrene pom-poms has only two steps and is free of PPM or purification, large amounts of up to 300 g of defined pom-pom structures can be synthesized in one batch.
1 Introduction
Block copolymers and branched structures with low polydispersity and architectural purity are of great interest not only to chemists, but also to physicists to refine or create new molecular theories, constitutive equations, and new materials with enhanced properties. The discovery of anionic polymerization by Szwarc[1] enabled polymer chemists to synthesize polymers of low dispersity and facilitated access to complex structures. Diblock copolymers, stars, and combs were prepared first, due to their simple nature.[2, 3] Soon after, the H-shaped or pom-pom topology got into the focus of synthetic chemists and polymer physicists. The pom-pom topology consists of two stars covalently connected by a single chain and is the simplest topology with exactly two branching points. Models and constitutive equations were developed to describe the melt flow behavior of the pom-pom in different flow fields, as an idealized branched molecule.[4, 5] Efforts were made to synthesize pom-pom-shaped polymers to further investigate their rheological behavior and compare them to the developed models.[6-11] The relation between the arm molecular weight Mw,a, the backbone molecular weight Mw,b, and the arm number q is decisive for their rheological relaxation mechanism. Different synthetic approaches were developed successfully, but most of them are limited in control over one of the molecular parameters. The use of multifunctional linkers as the branching points is popular due to the comparably good control over all molecular parameters.[12-16] However, commercially available linkers like Hexakis-(bromomethyl)benzene have up to six functional groups, which limits the arm number to five arms per pom as one functional group is needed to connect with the backbone.[17, 18] Another synthetic route involves the synthesis and polymerization of macromonomers for the stars of the pom-pom followed by monomer addition for the backbone and linking. Nonetheless, this convergent approach is prone to topological impurities by symmetric and asymmetric stars, and control and determination of the backbone molecular weight is difficult.[6, 8]
In comparison to pom-poms, the synthesis of star polymers is rather simple. Using multifunctional linkers and/or end-capping techniques, symmetric and asymmetric stars can be obtained by anionic polymerization[19-24] and more recently also with ring-opening and controlled radical polymerization.[25] For diblock copolymers, almost any combination of monomers and sequences can be obtained by sequential addition, linking, or end-functionalization techniques through anionic polymerization[26-28] and more recently through controlled radical polymerization[29, 30] resulting in a vast variety of properties.[31]
Thermoplastic elastomers (TPE) combine the mechanical properties of rubber with the processability of thermoplastics by using phase-separating block copolymers with different glass transition temperatures. For their synthesis, anionic polymerization is favored due to the fast access to high molecular weight block copolymers and defined topologies as a key to tuning mechanical properties.[32] TPEs and homopolymers are used in a wide application field spanning from packaging, construction, automotive, and electronics, to even medical applications and are projected to rise in the future.[33]
In this work, an easy and fast synthetic route to access a variety of branched topologies is shown. The P2VP-grafting route is a two-step synthesis consisting of the backbone polymerization containing small amounts (typically <2 mol% in the branched product) of poly(2-vinylpyridine) (P2VP) as branching points in the first step and polymerization and grafting of the arms onto P2VP blocks at the C6 carbon of the pyridine ring in the second step. Two exemplary cases are shown to highlight the versatility of the synthetic route towards a variety of styrene and diene-based monomers. In the first example, the P2VP-grafting route is used to access the pom-pom topology for PS with high arm numbers offering full control over all molecular parameters. In a second example, the synthesis of a PS-b-PI star with 8 and 15 arms is demonstrated to show the use of the P2VP-grafting route for the synthesis of TPEs. Due to its simplicity, the P2VP-grafting route is also suitable for upscaling, enabling the synthesis of up to 300 g of low dispersed, topological pure pom-pom-shaped PS in one batch within 3 days.
2 Synthesis
The presented approach allows facile access to densely grafted, branched architectures. As shown in Figure 1, the challenging pom-pom architecture can be obtained in a straightforward, two-step synthesis with full control over all molecular parameters. In the first reaction step, the backbone of the pom-pom (P2VP-b-PS-b-P2VP) is synthesized via a bifunctional initiator and sequential monomer addition of styrene and 2VP. The bifunctional initiator is generated by an electron transfer of sodium naphthalenide (NaNp) onto styrene monomer followed by immediate dimerization in tetrahydrofurane (THF) at −80 °C. The rest of the styrene and the 2-vinylpyridine are added sequentially to form the desired P2VP-b-PS-b-P2VP polymer. The P2VP blocks are kept short compared to the PS blocks (monomer ratio 2VP to styrene about 1:10) and the triblock can therefore be approximated as a PS chain with functional end groups. After termination, the solution is precipitated into methanol (MeOH) and the polymer is dried under reduced pressure. In the second reaction step, the arms of the pom-poms are attached to the backbone via grafting onto the C6 carbon of the pyridine ring. The PS arms are polymerized in cyclohexane through initiation by s-butyllithium (s-BuLi), resulting in a very narrow dispersity below Đ < 1.05, and then added to the backbone dissolved in THF at room temperature. The pom-pom product can be obtained after 16 h and is precipitated in MeOH and is named 300k-2 × 24–40k using its molecular properties with the scheme Mw,b-2q-Mw,a. The molecular parameters of the synthesized polymers can be found in Table 1. Unreacted PS arms are removed by fractionation from THF/MeOH easily due to the large difference in molecular weight.
| Sample | Mw [kg mol−1]a) | Đ | Purity [%]b) | P2VP content [mol%]c) | PS content [vol%]c) | q |
|---|---|---|---|---|---|---|
| Pom-pom 300k-2 × 24–40k | ||||||
| Backbone P2VP-PS-P2VP | 300 | 1.4 | - | 12.4 | ||
| Arms | 40 | 1.03 | - | 0 | ||
| Crude pom-pom | 2220 | 1.4 | 75 | 1.1 | 2 × 24 | |
| Fractionated pom-pom 300k-2 × 24–40k | 2220 | 1.4 | > 95 | 1.5 | 2 × 24 | |
| 15-Arm star: (SI)15-g-P2VP-26% | ||||||
| Backbone P2VP | 7.7 | 1.3 | - | 100 | ||
| Arms | 52 | 1.04 | - | 0 | ||
| Crude star | 788 | 1.08 | 70 | 1.2 | 15 | |
| Fractionated star | 788 | 1.08 | 92 | 1.7 | 26.0 | 15 |
| 8-Arm star: (SI)8-g-P2VP-28% | ||||||
| Arms | 55 | 1.05 | - | 0 | ||
| Crude star | 423 | 1.11 | 68 | 1.3 | 8 | |
| Fractionated star | 423 | 1.11 | 90 | 1.6 | 28.5 | 8 |
- a)Mw determined by MALLS for block copolymers, see experimental section for details;
- b)Estimated by integrals of DRI signals as shown also in ref. [43];
- c)Estimated by 400 MHz 1H NMR.
Similarly, a star-shaped PS-b-PI polymer with 15 arms by grafting-onto was obtained. Living PS-b-PI chains are synthesized by the initiation of styrene with s-BuLi in cyclohexane, followed by the addition of isoprene. The living PS-b-PI chains are then added to a short (in comparison to the arms) linear P2VP core dissolved in THF, as shown in Figure 1. The reaction is terminated with degassed MeOH after stirring for 16 h and precipitated in MeOH. Unreacted arms are removed by fractionation from cyclohexane/isopropanol. All molecular parameters are listed in Table 1. Both synthetic routes resulted in lab-scale batches of around 10 g of the purified product. Similar grafting-onto methods to stars described in the literature are typically limited to six arms, as the maximum functionality of typical linkers such as hexakis (bromomethyl)benzene.[34-37] The here synthesized PS-b-PI stars have 8 and 15 arms. These high arm numbers are else reported only by other grafting techniques, rarely by grafting-onto approaches.[38, 39] Using other polymerization techniques such as atom transfer radical polymerization (ATRP), multi-step synthetic procedures, and other monomers, higher arm numbers with up to 21 arms can be obtained.[40-42]
In Figure 2, the size exclusion chromatograms are shown for the pom-pom backbone, the crude and fractionated product. For the backbone, a narrow and monomodal weight-average molecular weight distribution is obtained. No partial termination of the living anions after the addition of 2VP or coupling products can be observed. The raw product of the grafting-onto step shows a distinct shift in elution volume from Ve,peak = 13.6 to Ve,peak 12.0 mL indicating increased molecular weight and therefore successful grafting. Around Ve = 16.0 mL, about 20% unreacted arms can be identified. After one fractionation of the crude product in THF/MeOH, the pom-pom compound can be obtained with high purity (< 5% residual arms).
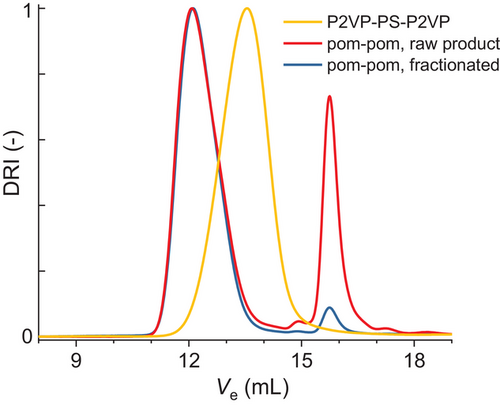
The total molecular weight of the pom-poms was confirmed by multiangle laser light scattering (MALLS).
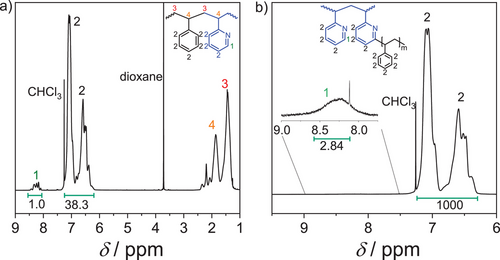
The 1H NMR spectrum of the P2VP-PS-P2VP backbone is shown in Figure 3a). The backbone monomer composition between PS and P2VP is estimated at 12.4 mol% P2VP as shown in Table 1. To confirm the molecular structure of the pom-pom estimated by the integrals of the SEC traces, the 1H NMR spectrum of the pom-pom was analyzed and is shown in Figure 3b). The multiplet between δ = 7.2 – 6.3 ppm can be assigned to the aromatic protons (Figure 3: 2) and the singulet around δ = 8.2 ppm, shown in the inlet, to the residual C6-H protons on the pyridine ring (Figure 3: 1). Calculating the integral ratios between the two peaks from the molecular architecture estimated by the SEC traces yields an integral ratio of 2.6:1000 (C6-H:C-Harom). Due to the low concentration of C6-H protons, the integral is partially overlapped by the neighboring peaks. To obtain the correct integral, a Gaussian function is fitted to the neighboring peak and its intensity is subtracted. The ratios determined in the NMR spectrum in Figure 3b) show a ratio of 2.84:1000. This confirms the molecular structure of the pom-pom with q = 2 × 24 within the margin of error. The grafting density of the herein-reported grafting procedure is around one out of five P2VP repeating units, similar to previously reported observations.[44]
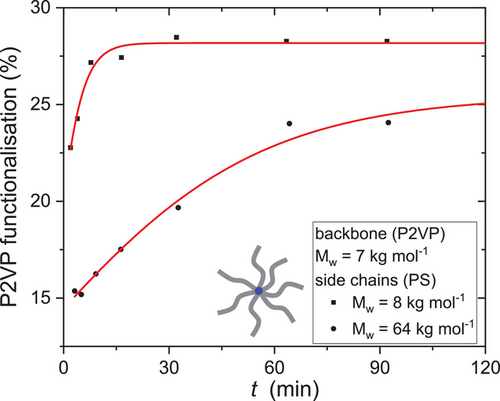
To investigate the kinetics of the grafting reaction, the same star backbone was grafted onto PS arms of Mw,a = 8 and 64 kg mol−1. Samples were taken after 3, 5, 9, 16, 32, 64, and 92 min after the addition of the arms to the backbone. The percentage of the functionalization of the P2VP repeating units is shown in Figure 4 as a function of the reaction time. Before the first sample was taken (t = 3 min), a major part of the grafting reaction was already completed. Between t = 3 min and t = 15 min for Mw,a = 8 kg mol−1 the reaction reaches its plateau value of 28% functionalization. For Mw,a = 64 kg mol−1, the reaction is slowed and reaches a lower plateau value of around 25% functionalization due to the longer arms, that is, larger hydrodynamic radii and space occupation around the P2VP backbone.
Due to the few reaction steps and the absence of post-polymerization modification reactions, this route is also suitable for large-scale synthesis. Using large round bottom flasks with up to 4 L, 300 g of product could be synthesized in one batch, with a total reaction time of 1 day. The reaction time could be further decreased by using more polar solvents as, for example, toluene or THF for the polymerization of the arms instead of cyclohexane.[26, 45] The generated heat from the polymerization is limiting the achievable reaction speed but can be controlled with adequate cooling. More polar solvents present will speed up the grafting reaction as well. Therefore, we estimate that at an industrial scale, the complete reaction could be achieved in several hours. The industrial use of the pom-pom topology as an additive or a high-performance compound utilizing the low viscosity and high strain hardening can be enabled through the reported synthesis. For example, recycled polymers often are subject to chain scission during mechanical recycling. The addition of small amounts of pom-poms polymers can increase the melt strength significantly to ensure good processability.[46] Both low viscosity and high strain hardening are also beneficial during the foaming of polymers for example for highly insulating foams or ultra-tough foams for body armor such as crash helmets.[47]
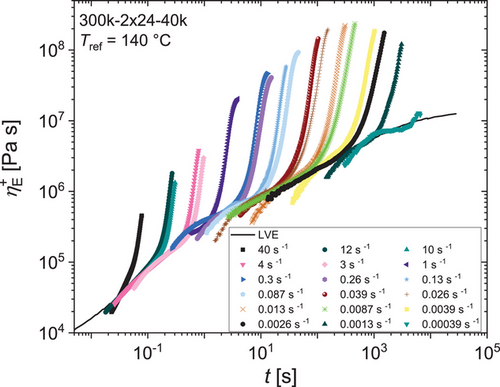
Branched topologies with more than one branching point are known to exhibit strain hardening in elongational melt flow.[8, 43, 48-53] Pom-poms being the simplest topology with at least two branching points show strong strain hardening in extensional flow as shown multiple times.[8, 43, 54] To confirm the successful grafting reaction rheologically, we investigated the time-dependent tensile stress growth coefficient of the pom-pom 300k-2 × 24–40k. Tensile stress growth coefficients for strain rates between = 0.00039 − 40 s−1 are shown in Figure 5 at a reference temperature of Tref = 140 °C. Over the whole range of strain rates, high-strain hardening can be found. The highest extensional viscosity ηE can be found for = 0.0087 s−1 with ηE = 234 MPa s. The strain hardening factor (SHF) is used to easily quantify and compare the strain hardening of polymer melts. It is defined as the ratio of the measured extensional viscosity ηE to the prediction of the Doi–Edwards model ηDE by . For example, commercial low-density polyethylene (LDPE) has a SHF ≈ 10. The herein synthesized pom-pom 300k-2 × 24–40k has maximum strain hardening factor SHFmax = 112 (at = 0.026 s−1, Tref = 140 °C). Combs with similar molecular parameters reach only SHFmax ≈ 90 with considerably higher synthetic effort utilizing three reactions and several workup steps compared to two reaction steps without further workup steps.[48, 55, 56]
For the PS-b-PI-g-P2VP stars, the linear precursors and the stars show a narrow and monomodal molecular weight distribution (see Figure S1, Supporting Infromation). High 1,4-microstructure content is obtained for the PI-block, as expected from the polymerization in hydrocarbons (see Figures S2 and S3, Supporting Information). In the crude product of the 15-arm star, about 30% of unreacted arms can be identified around Ve = 15.2 ml and small amounts of dimerized arms can be found around Ve = 14.2 ml, most likely resulting from contamination of the living arms with oxygen during the grafting reaction. After a single fractionation of the crude product from cyclohexane/isopropanol, high purity of the star (92 %) could be achieved.
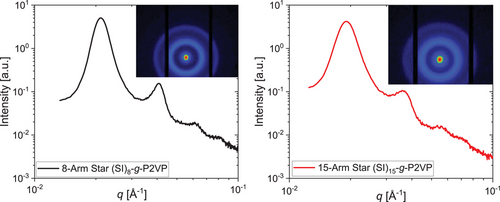
Small-angle X-ray scattering (SAXS) measurements were performed on the two PS-b-PI samples to investigate their morphology. Peak sequences of the two stars are shown in Figure 6 revealing asymmetric lamellar morphologies (1q0:2q0:3q0:4q0) for both stars. In contrast, diblock[57] and triblock copolymers[58, 59] with similar PS/PI volume fractions around 28 vol% PS show a cylindrical morphology. Herein, the influence of the molecular architecture is visible. Similar long-range order distances can be obtained for the two stars with L0 = 29.6 nm for the 8-arm star and L0 = 32.7 nm for the 15-arm star.
In Figure 7, the uniaxial tensile stress-strain curves of the PS-b-PI-g-P2VP stars are shown. The stress response is governed by a linear regime up to a strain of ε 2% and yielding around ε ≈ 3–4%. A broad yielding region is shown up to ε = 500 % and then strain hardening up to the ultimate tensile strength σU up to σU = 20.8 ± 0.8 MPa for the 15-arm star and σU = 21.0 ± 3.8 MPa for the 8-arm star can be observed. The samples rupture at εbreak = 1106 ± 36 % for the 15-arm star and εbreak = 971 ± 58 % for the 8-arm star.
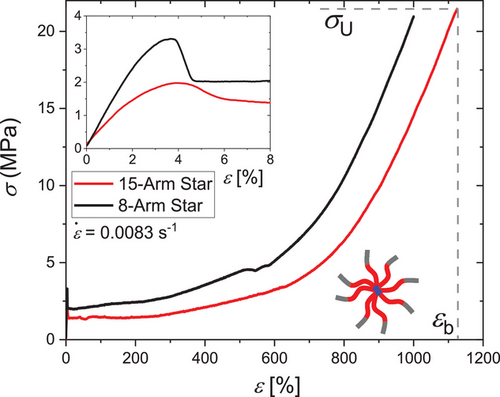
When comparing σU of different architectures across literature, a benchmark of about σU = 20 MPa emerges, which is surpassed only rarely. Most architectures reach their maximum σU σUof ≈15 MPa. Both herein-reported stars surpass the 20 MPa benchmark, outperforming other samples with related complex architectures but much higher synthetic effort like miktoarm[37] and tapered stars[34] and with different architectures like symmetric[60, 61] or asymmetric-linear,[37] tapered,[62] and multigraft copolymers.[61, 63]
Beyond the synthesis of star and pom-pom-shaped polymers with full control over all molecular parameters, the P2VP-grafting route allows facile access to many quasi-homo- and block copolymer topologies with high grafting densities. Further possible topologies are shown in Figure 8, such as comb or barbwire type topologies, miktoarm stars, or Janus polymers, underlining the wide range of possible applications of this synthetic route. Although these topologies can also be accessed through other synthetic routes as well, some lack control over the molecular parameters, require time- and work-intensive break-seal techniques, and usually are only suitable for small-scale synthesis in the lab (up to ≈10 g). Using the P2VP-grafting route, the unparalleled properties of well-defined, branched topologies can be enabled for large-scale use in high-performance consumer products. For example, the combination of low melt viscosity and high strain hardening of pom-poms could be used as an additive to ensure the processability of a polymer compound that suffers from chain degradation after mechanical recycling. Obvious is also the use of styrene-b-isoprene copolymers as arms to synthesize branched TPEs.
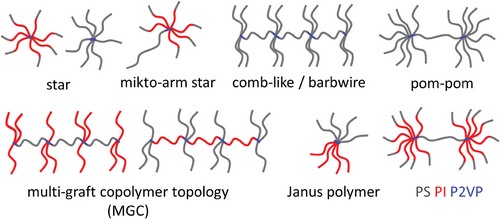
Block copolymers of styrene and isoprene are easily accessible by sequential addition in any ratio and sequential order. Like homopolymers, branched structures block copolymers allow tuning and significantly increase mechanical properties like εbreak or σUTS compared to di- or triblock copolymers.[64] Even though many different architectures and their effects on the mechanical properties of TPEs have been investigated, the commercial application is limited by the complex synthesis as well. Scale up through the P2VP-grafting route should be rather simple, particularly for symmetric, asymmetric, and miktoarm stars, and enable the use as high-performance TPEs or as additives, even at an industrial scale.
Furthermore, the P2VP-grafting route can be used for the synthesis of Janus polymers. Through multiple, sequential grafting steps different arms can be attached. The only limiting factor is the reactivity of the C6 carbon of the pyridine ring requiring highly reactive carbanions for the grafting reaction.
3 Conclusion
Branched structures with low polydispersity and architectural purity are of great interest not only to polymer chemists but also to polymer physicists to refine or create new theories and to develop new materials with superior properties. In this work, a new synthetic approach to easily access densely grafted, branched topologies of styrene and butadiene derivates with full control over all molecular parameters and low dispersity is shown. As a first example, the two-step P2VP-grafting route is used to access a PS pom-pom with very high arm numbers of 2 × 24 arms. The branched topology could be confirmed rheologically by the observed strong strain hardening in elongational flow as a rheological fingerprint of branching. In the second example, PS-b-PI stars are synthesized and the mechanical properties are investigated to show the application as thermoplastic elastomers. In addition, we discuss further synthetic possibilities of the P2VP-grafting route towards the fast access with high grafting densities of other branched homo- and block copolymer structures like barbwire, bottlebrush, mikto-arm star, Janus polymers, combs, and multi-graft copolymers. Due to the synthetic simplicity and fast completion within minutes of the P2VP-grafting route, it is also suitable for upscaling beyond the laboratory scale. Up to 300 g of low disperse and architecturally well-defined pom-poms could be synthesized in one batch typically within three days.
4 Experimental Section
Materials
Styrene (99% extra pure, stabilized, Sigma-Aldrich), 2-vinylpyridine (97%, stabilized, Fisher Scientific), and 1,1-diphenylethene (Sigma-Aldrich) were stirred over calcium hydride (CaH2, 92% Fisher Scientific) overnight, degassed by three freeze-pump-thaw cycles, and freshly distilled prior to use. Isoprene (98%, stabilized, VWR) was stirred in an ice bath over n-butyllithium (2.5 M in cyclohexane, Sigma-Aldrich). As soon as the solution turned slightly yellow, isoprene was distilled into ampules. Cyclohexane (99%, Fisher Scientific) was purified by stirring over polystyrenyllithium, degassed by three freeze-pump-thaw cycles, and freshly distilled prior to use. Tetrahydrofuran (THF, 99.5%, Roth) was distilled from CaH2, stored over sodium/benzophenone, degassed by three freeze-pump-thaw cycles, and freshly distilled before use. Methanol (>99%, Fisher Scientific) was degassed by three freeze-pump-thaw cycles. S-butyllithium (s-BuLi, 1.4 M in cyclohexane, Aldrich), iso-propanol (>99%, Fisher), sodium (>99%, Merck), naphthalene (99%, Acros), THF (HPLC grade, Fisher), 1,4-dioxane (>98%, Fisher), and benzophenone (Sigma-Aldrich) was used as received.
The SEC instrument was an Agilent 1200 series equipped in a four-detector configuration with UV, multiangle laser-light scattering (MALLS, SLD7000/BI-MwA [PSS, Brookhaven Instruments]), viscometer, and a DRI detector (Polymer Standard Service, PSS, Germany), using an autosampler for sample injection. The columns (SDV-Lux 5 µm (guard column), SDV-Lux-1000 Å, SDV-Lux 100000 Å, PSS) were calibrated with monodisperse linear PS standards with weight-average molecular weights, between 476 and 2.5·106 g mol−1. HPLC grade THF was used as the eluent with a flow rate of 1 ml min−1. 100 µl was injected for each measurement. dn/dcvalues were calculated based on the ratio of the blocks (mol.%) as determined by 1H NMR.
1H NMR spectra were obtained in deuterated chloroform at 25 °C using a 400 MHz Bruker Avance III Microbay spectrometer.
Stress-strain curves were measured on a Hegewald–Peschke inspect table 10 kN in uniaxial testing at a linear strain rate of 0.0833 s−1 with dog bone specimens cut from a film of the samples (DIN 53504). Polymer films were obtained from (THF) solution evaporation at room temperature with subsequent drying at 120 °C under vacuum for 4 h.
Rheological data was obtained on a TA Instruments ARES G2 with 13 mm parallel plate or extensional viscosity fixture (EVF) geometry. Samples were hot pressed at 180 °C for 15 min under vacuum.
Microstructural information about the PI and PS microdomains in the solid samples was obtained by SAXS on a Xeuss 2.0 Q-Xoom, Xenocs SA, Grenoble, France, q = 0.001 − 4 nm−1.
Experimental Procedure for the Synthesis of the Pom-Pom
All polymerization steps were conducted under inert Schlenk conditions.
Experimental Procedure for the Synthesis of the Pom-Pom—Synthesis of the Backbone
4 mL NaNp solution (0.05 M, 0.2 mmol) was added to 1000 mL of freshly distilled THF at −80 °C. 58.6 mL Styrene (53.3 g, 0.512 mol) was added and the polymerization was allowed to proceed for 20 min. 6.9 mL 2-Vinylpyridine (6.72 g, 0.064 mol) was added and the polymerization was terminated by 1 mL MeOH after 5 min. The polymer was precipitated from MeOH and freeze-dried from 1,4-dioxane solution.
Experimental Procedure for the Synthesis of the Pom-Pom—Synthesis of the Arms
50 ml styrene was added to 2.5 L of cyclohexane and initiated by 5.1 mL s-BuLi solution (1.4 M, 7.34 mmol). Styrene was added gradually over 10 h (Total styrene: 285.4 g, 2.74 mol) for the temperature of the reaction mixture to be around 30 °C.
Experimental Procedure for the Synthesis of the Pom-Pom—Grafting Reaction
32.5 g dried backbone was dissolved in 300 mL THF. Living arms were added and the reaction was stirred for 16 h at room temperature. Residual arms were terminated with 1 mL degassed MeOH. The polymer was precipitated from MeOH and fractionated with MeOH/THF.
Experimental Procedure for the Synthesis of the 15-Arm Star—Synthesis of the Backbone
5.13 mL of freshly distilled 2-vinylpyridine (5.00 g, 47.6 mmol) was added to 150 mL of freshly distilled THF at −80 °C. 0.71 mL of s-BuLi solution (1.4 M, 1 mmol) was added and the polymerization was terminated after 10 min by the addition of 1 mL of degassed MeOH. The polymer was precipitated from MeOH and freeze-dried from 1,4-dioxane solution.
Experimental Procedure for the Synthesis of the 15-Arm Star—Synthesis of the Arms
6.7 mL styrene (6.0 g, 57.6 mmol) was added to 150 mL of cyclohexane, followed by 0.29 mL s-BuLi solution (1.4 M, 0.4 mmol). After stirring 16 h at room temperature, 20.6 mL isoprene (14.0 g, 205 mmol) was added and stirred for 16 h.
Experimental Procedure for the Synthesis of the 15-Arm Star—Grafting Reaction
0.133 g P2VP backbone (1 eq.) was dissolved in 100 mL THF and the living arms (15 eq.) were added and stirred for 24 h. Residual arms were terminated with 1 mL MeOH. The polymer was precipitated from MeOH and fractionated with iso-propanol/cyclohexane.
Acknowledgements
The authors thank Michael Pollard for proofreading the manuscript as a native English speaker. Furthermore, the authors would like to thank Prof. Manfred Wilhelm for all the support, especially the access to the great infrastructure and working environment available, as well as for highly fruitful discussions. The authors express thanks to Prof. Hermann Nirschl and Simon Buchheiser for SAXS measurements.
Open access funding enabled and organized by Projekt DEAL.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




