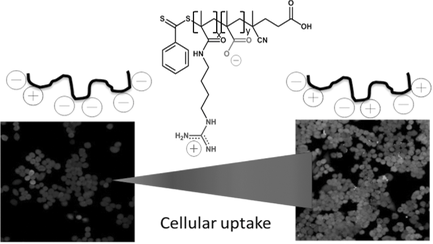
Direct Correlation Between Zeta Potential and Cellular Uptake of Poly(methacrylic acid) Post-Modified with Guanidinium Functionalities
Abstract
Cellular uptake is identified as one of the key parameters to the successful delivery of drugs into cancer cells. In this study, the effect of the ratio of negative and positive charges on a polymer on the amount of uptake is studied. Poly(methacrylic acid), prepared by RAFT polymerization, is post-functionalized with agmatine in water to various degrees. When agmatine ratio is increased to 1, the conjugation efficiency is observed to be ≈63% indicating potentially some steric hindrance or excessive repulsion by the positive charged guanidinium functionality. The resulting zeta potential, ranging from −40 to −14 mV, is in linear correlation with the degree of functionalization. The resulting polymers are found to be non-toxic to A2780 ovarian cancer cells. The cellular uptake is subsequently studied by laser scanning confocal fluorescent microscopy, flow cytometry, and by analysis of the fluorescence intensity of the remaining solution after incubation with A2780 ovarian cancer cells. An almost linear relationship between zeta potential and cellular uptake is observed.
1 Introduction
The delivery of drugs using nanoparticles and polymers is expected to be able to address issues such as low drug stability, side effects, and drug resistance issues.1 The cellular uptake of the drug carrier by cells is often identified as a key parameter to the efficient action of drugs. A high cellular uptake is often in directly correlates with the toxicity of the drug.2 It is therefore not surprising that copious reports are available that investigate the influence of size, shape, and surface chemistry of the drug carrier on drug delivery.3 The cellular uptake of nanoparticles and polymers is directed by a two-step process. The initial surface attachment to the cellular membrane is followed by the internalization step, usually by endocytosis. It is therefore plausible that the surface chemistry of the polymer nanoparticles will play a pivotal role in cell uptake as it is the interface to the biological world. The composition of the cell surface is dominated by negatively charged species such as heparan sulfate proteoglycans, but also other negatively charged compounds such as phosphatidylserine and anionic phospholipids are present.4 It is therefore not surprising that cell-penetrating peptides (CPPs)5 that are rich in the positively charged arginine are frequently used to enhance the entry into cells. Decoration of nanoparticles with Tat peptide6 or oligoarginine7 was frequently used to enhance the delivery of nanoparticles, but a similar effect can be observed when nanoparticles are coated with cationic polymers such as chitosan[1] and polyethylene imine.8
Inspired by CPPs with their abundance of arginine, researchers have mimicked these biological molecules using guanidinium-rich polymers.9 Attachment of guanidinium functionalities to polymers, which coincides with the introduction of positive charges along the polymer, can significantly enhance the cellular uptake.9 The arrangement and distribution of cationic groups along the polymer can have detrimental effects on the properties with arginine residues bundled in a block-like structure showing superior properties to evenly distributed charges along the chain.10 Moreover, a direct relationship between the zeta potential, which is one measure for the surface charge, and the cellular uptake has been reported.11
Despite the frequent use of cationic polymers to enhance cellular uptake, concerns in regards to their cytotoxicity remain. The high toxicity of these polymers even facilitates their use as antimicrobial agents.12 As a result, the design of drug delivery carriers based on cationic polymers needs to be carefully planned. Recently, a zwitterionic polymer based on arginine displayed a high cellular uptake but no cytotoxicity.13 The balance of negative and positive charges led the formation of an almost neutral polymer. However, the polymer still displayed enhanced cellular uptake while the polymer was non-toxic even at high concentrations. Although the mechanism of the interaction of this polymer with cells is unknown, the observation may suggest that polymers carrying positive and negative charges may display overall superior properties as drug carriers.
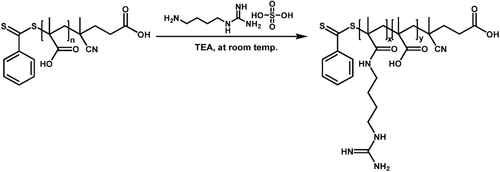
In this paper, a systematic study was put in place that seeks to answer the question if there is a direct correlation between the zeta potential of guanidinium-functionalized polymers and the cellular uptake. Therefore, poly(methacrylic acid) will be post-functionalized with agmatine to generate polymers with various ratios of cationic and anionic functional groups (Scheme 1). Agmatine is a guanidine derivative formed by decarboxylation of arginine, which was previously attracted attention as an endogenous ligand for the I1 receptor, which is located on the plasma membrane.14 The structural feature of agmatine is essential for enhanced cellular uptake due to its guanidine group, which can interact with the phosphate or other anionic moieties on the cell surface. Therefore, several reports have described the preparation of chitosan–agmatine bioconjugates, dextran–agmatine bioconjugates, and polyamidoamine bearing agmatine for efficient drug delivery system.15
In contrast to earlier work,16 the two charges are not present as zwitterionic structure, but they are randomly distributed along the polymer chain. The aim of this study will be to identify the effect of the fraction of agmatine on the toxicity toward A2780 ovarian cancer cells and to evaluate the relationship between the net surface charge of the polymer and the cellular uptake.
2 Experimental Section
2.1 Materials
Methacrylic acid (Aldrich, 99%), fluorescein O-methacrylate (Aldrich, 97%), fluorescein isothiocyanate (FITC) (Aldrich, ≥90%), agmatine sulfate salt (Aldrich, ≥97%), dioxane (Ajax: 99%), anhydrous di(ethyl ether) (Adrich, ≥99%), triethylamine (TEA) (Scharlab, 99%), and sodium azide (Aldrich, ≥99%) were used without any further purification. 2,2-Azobisisobutyronitrile (AIBN) (Fluka; 98%) was recrystallized from methanol. Other chemicals and solvents were used as received. Milli-Q water was produced by a Milli-Q reverse osmosis system and its resistivity was 18 MΩ cm. The RAFT agent, 4-cyanopentanoic acid dithiobenzoate (CPADB) was synthesized according to the literature.17 Deuterated solvents for NMR were used as received.
2.2 Synthesis and Procedures
2.2.1 Synthesis of Poly(methacrylic acid) PMAA via RAFT Polymerization
The polymerization was conducted using chain-transfer agent 4-cyanopentanoic acid dithiobenzoate (CPADB). For the polymerization, MAA (1 g, 11.6 mmol), CPADB RAFT agent (32 mg, 0.116 mmol), the initiator AIBN (2 mg, 0.0116 mmol), fluorescein O-methacrylate (9.3 mg, 0.023 mmol) as well as 1.5 mL of dioxane were combined in a Schlenk flask equipped with a magnetic stirrer bar. The glass flask containing mixture was sealed with a glass stopper and degassed by three freeze-pump-thaw cycles, back filled with nitrogen. The polymerization was conducted for 3 h at a temperature of 70 °C. Then the polymerization solution was terminated by introducing air to the mixture and placing the flask in an ice water for 15 min. The resulting poly(methacrylic acid) was collected by precipitating in anhydrous diethyl ether and centrifuged at 7000 rpm for 10 min. After purification, the polymer was dried in a vacuum oven for 2 d. Monomer conversion was determined by 1H NMR (D2O) (conversion = 60%). The peaks observed at δ = 5.63–6.1 ppm (2H) correspond to the protons from MAA residual monomer and signals at δ ≈ 0.6–1.4 ppm (3H) and at δ = 1.92 ppm (2H) indicates the protons in PMAA. Molecular weight and molecular weight distribution (Mn = 111 300 g mol−1, Ð = 1.19) were obtained by SEC with water as eluent (flow rate = 0.8 mL min−1, temperature = 30 °C) using PEG/PEO standards.
2.2.2 Agmatine Conjugation to PMAA
The conjugations of amine-containing ligands to PMAA were carried out at room temperature without using any additional coupling agents. The guanidinium containing copolymers were prepared using molar ratios of agmatine and carboxylate groups from 0 to 1. The reaction using [COOH]0:[Agm.]0:[TEA]0 = 1:1:2.5 was carried out as follows: in a 10 mL round bottom flask equipped with a magnetic stirrer bar, PMAA (25 mg) was dissolved in Milli-Q water (2.8 mL). After complete dissolution, the mixture of agmatine (66 mg, 0.29 mmol) and TEA (73.5 mg, 0.726 mmol) dissolved in Milli-Q water (2.2 mL) was added to the reaction dropwise. Then the mixture was left stirring for 24 h at room temperature. The final products were purified by dialysis against Milli-Q water using the 3500 MWCO dialysis bags for 3 d in order to remove the excess ligands and lyophilized for 2 d. The ratio of agmatine to TEA was kept at 1:2.5 for all conjugation reactions. The successful amine conjugation was confirmed by 1H NMR (D2O) from the agmatine peaks: δ = 2.984 ppm (2H), δ = 3.2 ppm (2H) and δ = 1.65 ppm (5H). Molecular weight and molecular weight distribution of amine-conjugated polymers were obtained by SEC with water as eluent (flow rate = 0.8 mL min−1, temperature = 30 °C) using PEG/PEO standards.
2.2.3 Synthesis of FITC-Labeled Amine-Conjugated Polymers
FITC was chemically attached to the polymer after post-modification using a molar ratio between polymer and FITC of 1: 2. First, 5 mg of polymer was dissolved in 1 mL of Milli-Q water and then the stock solution of FITC in DMSO was added dropwise. Then, the reaction was left overnight in dark. Finally, the FITC-labeled polymers were dialyzed against Milli-Q water for 2 h. The fluorescence intensity of the polymers is presented in Table 1.
| PMA(Agm.)x-PMA(A)y | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Sample | [MAA] : [Agm.] | Conjugation efficiency [%]a) | x | y | Mn (theo) [g mol−1]b) | Mn (SEC) [g mol−1]c) | Ð | Fluorescence intensity [a.u.]d) | ZP [mV]e) |
| Agm.0 | – | – | 60 | 0 | 5440 | 111 300 | 1.19 | 446 | −40 |
| Agm.12 | 1:0.2 | 100 | 48 | 12 | 6790 | 113 000 | 1.13 | 306 | −26 |
| Agm.21 | 1:0.35 | 100 | 39 | 21 | 7800 | 128 300 | 1.08 | 222 | −23 |
| Agm.38 | 1:1 | 63 | 22 | 38 | 9710 | 131 700 | 1.07 | 182 | −14 |
- a)Ratio of agmatine attached/agmatine in reaction mixture;
- b)calculated based on PMAA60 and the amount of agmatine attached according to 1H NMR analysis;
- c)SEC analysis in water using PEG/PEO standards;
- d)fluorescence intensity after reaction with FITC;
- e)zeta potential analysis using DLS.
2.3 Cell Culture
Human ovarian cancer cell lines A2780 were cultured in tissue culture flasks using RPMI1640 media supplemented with 10% fetal bovine serum (FBS). The cells were grown at 37 °C under a 5% CO2 atmosphere. After reaching a confluent state, cells were detached by treating with Trypsin/EDTA.
2.4 Cytotoxicity Tests
The cytotoxicity tests were performed by a standard sulforhodamine B colorimetric proliferation assay (SRB assay). The ovarian cancer cell lines A2780 were seeded in 96-well cell culture plates at a density of 4000 cells per well for 24 h. The polymers were sterilized by filtration through 0.45 μm sterile membrane in bio-safety cabinet and then the growth medium was replaced with 100 μL fresh 2 × concentrated medium. Subsequently, 100 μL of serially diluted (2 × dilution) polymers were added to the plates, followed by incubation at 37 °C for 48 h.

2.5 Laser Scanning Confocal Microscopy
Cells were seeded in 35 mm Fluorodishes (World Precision Instruments) with a density of 2 × 105 cells per dish in 2 mL of medium and cultured for 2 d. The polymers were loaded to the cells at a working concentration of 200 μg mL−1 and incubated in a humidified incubator (5% CO2/ 95% air atmosphere at 37 °C) for 1 h. After incubation, the cells were washed with PBS three times. A laser scanning confocal microscope system (Zeiss LSM 780) consisted of, an argon laser (excitation wavelengths: 488 nm) connected to a Zeiss Axio Observer.Z1 inverted microscope (×20/1.4 NA objective ) was used for observation. The Zen2012 imaging software (Zeiss) was used for imaging acquisition and processing. The flouorescence intensities were analyzed by ImageJ.
2.6 Flow Cytometry
Cells were seeded in a six-well tissue culture plate at a density of 5 × 105 cells per well and cultured for 2 d. The polymers were loaded to the cells at a working concentration of 200 μg mL−1 and incubated in a humidified incubator (5% CO2/ 95% air atmosphere at 37 °C) for 1 h. After incubation, the cells were washed thrice with cold PBS. The cells were treated with trypsin, centrifuged, and resuspended in cold serum-free medium before measurements. The flow cytometry assay was performed with BD FACSCanto II Analyzer (BD Biosciences, San Jose, USA).
2.7 Cellular Uptake Analysis Using Fluorescence Spectrophotometer
The cellular uptake at different time intervals was measured by using fluorescence spectrophotometer. Cells were seeded in 24-well cell culture plates at a density of 1.5 × 105 cells per well and cultured for a day. The polymers were loaded to the cells at a working concentration of 500 μg mL−1 and incubated in a humidified incubator (5% CO2/95% air atmosphere at 37 °C). At predetermined time intervals, the supernatant was taken out for the measurement of fluorescence intensity. Then, 0.5 mL of fresh 1× concentrated RPMI was added to each well in order to keep the cells alive while measuring the fluorescence intensity.
2.8 Characterization
2.8.1 Nuclear Magnetic Resonance Spectroscopy
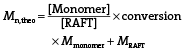 (1)
(1)2.8.2 Size Exclusion Chromatography
Size exclusion chromatography analyses were performed in Agilent modular system, comprising of an auto-injector, a PL aquagel-OH 8 μm bead size guard column (50 × 7.5 mm), two PL aquagel-OH MIXED-M 8 μm bead size columns (300 × 7.5 mm), a differential refractive index detector and a UV detector (λ = 305 nm). 0.02% w/v sodium azide solution in Milli-Q water was used as eluent at a flow rate of 0.8 mL min−1 at 30 °C. The apparent molecular weights and polydispersities were determined with a calibration based on linear polyethylene glycol/polyethylene oxide (PEG/PEO) standards with narrow molecular weight distributions. The molecular weights of standards are ranging from 106 to 1.5 × 106 g mol−1.
2.8.3 Fluorescence Spectrophotometer
Cellular uptake studies at different time intervals were carried out using Cary Eclipse fluorescence spectrophotometer. The excitation and emission wavelengths were 495 nm and 519 nm.
2.8.4 Dynamic Light Scattering
Zeta potential measurements were performed on a Malvern Zetasizer Nano ZS (He−Ne laser 633 nm, max 5 mW) with a light scattering angle of 173° and then the data were recorded by Malvern Zetasizer Software version 6.20. Samples in the concentration of 1 mg mL−1 were used for analysis without filtration. The measurements were run for three times at 25 °C.
3 Results and Discussion
MAA was polymerized in the presence of CPADB as a RAFT agent and AIBN as the initiator. The 1H NMR spectrum and SEC analysis of PMAA, shown in Figure 2 and Figure 3, respectively, confirmed that PMAA was successfully synthesized with a good control over the dispersity. After 3 h of polymerization, 60% of MAA was consumed and a monomodal distribution was obtained resulting in PMAA of 60 repeating units and a Mn = 111 300 g mol−1 (SEC) and Ð = 1.19, according to aqueous SEC using PEG/PEO standards.
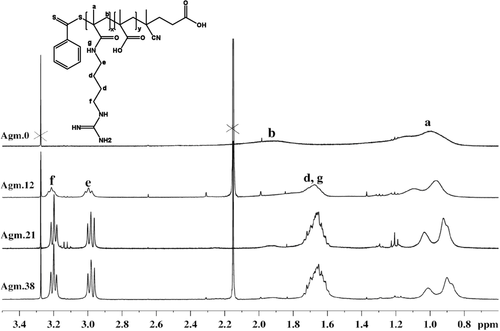
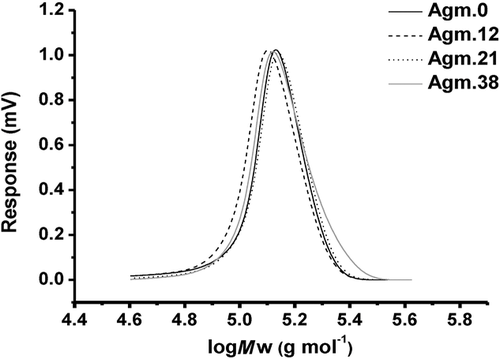
The aim of this work was the design of polymers with negative and positive charged functionality at various ratios to identify the relationship between the overall charge and the cellular uptake. Post-functionalization with agmatine was identified as a suitable means to introduce both functionalities as this can ensure even distribution of both charges along the chain. Initial attempts included post-functionalization of activated PMAA such as poly(N-succinimidyl methacrylate). However, the disparate solubilities of the lipophilic polymer and the hydrophilic agmatine only led to negligible yields due to the absence of a common solvent. Therefore, PMAA was directly functionalized with various amounts of agmatine in water (Table 1). This experiment was carried out without using any coupling agents such as EDC/NHS since the modification of COOH group with NHS ester, which is the typical intermediate in this reaction, may lead to the insolubility of products in water. After a reaction time of 24 h, the polymers were purified by dialysis against water. The degree of agmatine conjugation was determined by 1H NMR analysis in D2O (Figure 2). The methylene functionalities at around 2.98 and 3.2 ppm, respectively, are indicative of the successful reaction with agmatine.18 The degree of side chain substitution was determined from the peak of methyl group in polymer backbone (δ ≈ 0.6–1.4 ppm) to the side chain group, and is referred to as a percentage value. At low agmatine concentration, the agmatine conjugation reached 100%. However, an excess of amine could not improve the maximum agmatine content of around 60 mol% indicating that steric hindrance may play a role. In addition, the introduction of more cationic charges may lead to repulsion along the polymer chain. Repulsion may lead to unfavorable chain stretching while a conjugation efficiency of around 50% leads to a balance of negative and positive charges. Molecular weight analysis using SEC revealed a monomodal distributions and low dispersity (Table 1 and Figure 3). The molecular weight is significantly higher than the expected value as a result of the analysis in water and the used PEG/PEO standards. Moreover, according to the previous study, it was reported that SEC was found to be the least accurate than other common laboratory methods such as 1H NMR and MALDI-TOF in the determination of the molecular weight of polymers.19 Nevertheless, the SEC analysis shows that side reactions such as crosslinking reactions are absent as evident by the narrow-molecular-weight distribution.
The polymers were analyzed in water and 0.1 m NaCl using DLS. Hydrodynamic diameters below 5 nm confirm the existence of single polymer chains indicating that aggregate formation despite the presence of negative and positive charges is absent. Analysis of the zeta potential revealed a direct relationship between the amount of guanidine functionalities and the surface charge. It is interesting to note that in Milli-Q water the polymer Agm.38 has a slight negative charge although more guanidinium functionalities are present.
3.1 Cellular Uptake and In Vitro Cytotoxicity of Polymers
Human ovarian cancer cells (A2780) were chosen for the biological evaluation. Initially, the cytotoxicity of the agmatine-conjugated copolymers listed in Table 1 was tested using sulforhodamine B assay (SRB assay). Polymers obtained via post-functionalization modification were shown to be nontoxic. The negative charged carboxylate functionalities are therefore counterbalancing the typically toxic cationic charges. This is in agreement with earlier reports where zwitterionic polymers prepared using an arginine-based monomer showed no toxicity.13 In contrast to this report where negative and positive charges are distributed along the polymer backbone, zwitterionic polymers carry both charges in adjacent positions. According to Figure S1 (Supporting Information), it is illustrated that all polymers were found to be nontoxic to cells up to concentrations of 200 μg mL−1.
Following the in vitro cytotoxicity, the uptake of the post-functionalized polymers was monitored in order to evaluate the effect of arginine groups and the increasing positive charge. The polymers were therefore incubated with A2780 cells for 1 h and the uptake was monitored using laser scanning confocal microscopy and flow cytometry (Figures 3-5). Both techniques reveal that the fluorescence-labeled polymers were taken up (Figures 3-5). Interestingly, the increase in polymer uptake was in almost linear fashion with the amount of conjugated agmatine. The cationic-charged polymers possess a higher uptake because the cell membrane is anionic as a result of the anionic head group of phospholipids and the presence of carbohydrates such as sialic acid.20
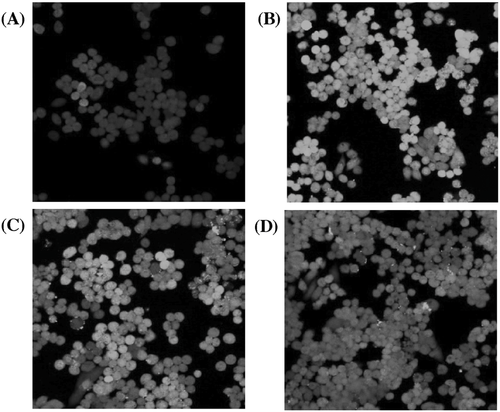
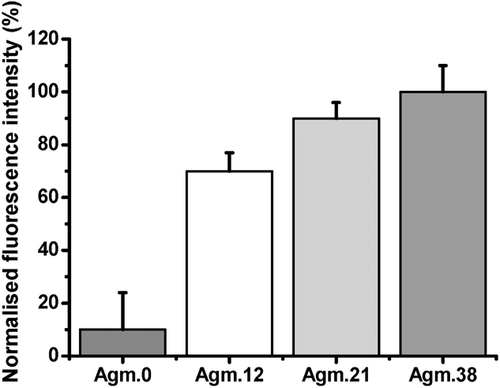
The results obtained from flow cytometry were subsequently normalized by taking the individual fluorescence intensity (Table 1) into account. As illustrated in Figure 6, PMAA exhibits poor uptake, but still higher than that measured by confocal microscopy. Although fluorescent microscopy can be considered more reliable than flow cytometry,21 the differences may not be as significant taking the error bars into account. Another possibility is that flow cytometry only measures the overall fluorescence of each cell, which may lead to errors from the polymer that is absorbed on the surface of the cell, but not internalized by the cells. In contrast, confocal microscopy will be able to distinguish the fluorescence caused by absorbed polymer and polymer in the cytosol.21
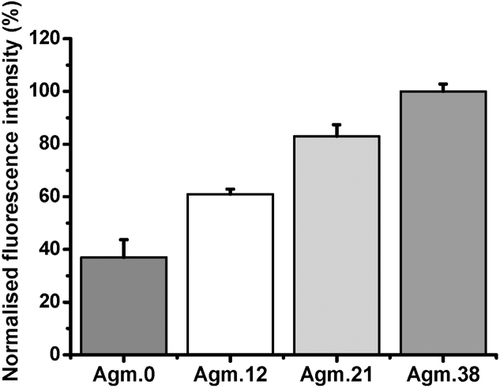
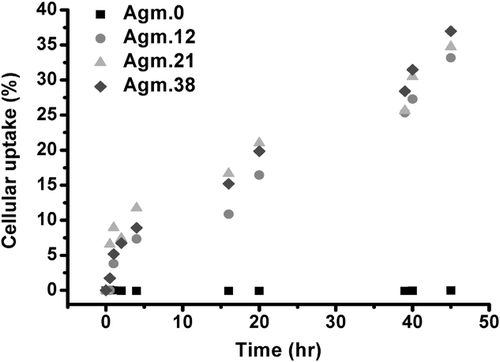
Moreover, the remaining solution that was used to culture the cells was analyzed and the decline in fluorescence intensity was recorded as a measure of the absorbed or internalized polymer. The internalization analysis over a period of time indicated around of 40% of the original agmatine conjugated polymers was taken up in a period of 45 h, while there was little uptake for PMAA (Figure 1). These results show little internalization of PMAA, which agrees with the confocal microscopy analyses. Interesting is the behavior of the agmatine-functionalized polymers over a period of 45 h. Although flow cytometry and laser scanning confocal microscopy suggest a slightly higher uptake of Agm.38 after 1 h, the differences between any of the modified PMAA polymers seem negligible when monitored over a long period of time. In overall, the cell uptake analyses demonstrated the efficient uptake of homopolymers with arginine functionality, and some internalization of PMAA, which might be as a result of the interaction of anionic polymer with cationic lipid domains in the cell membrane.20
4 Conclusions
We successfully synthesized polymers bearing both COOH and guanidinium functionalities. The polymers were incubated with A2780 human ovarian cancer cells at 37 °C in order to investigate in vitro cytotoxicity and cell uptake. The cytotoxicity analyses proved that the guanidinium-functional polymers did not introduce any toxicity, most likely due to the presence of negatively charged carboxylate functionalities. The polymers conjugated with agmatine were rapidly taken up by the cells. The increasing amount of guanidinium functional groups in the polymer chain led to a less negative zeta potential, which led to a highly efficient uptake. In contrast, PMAA itself was internalized to a negligible amount only. Interestingly, although higher agmatine functionalization led to a slightly higher uptake after 1 h, monitoring the cell uptake over a longer period of time suggests that there is only a small advantage of having higher guanidinium content. However, it can be concluded that the efficient cellular uptake and virtually nontoxic nature of agmatine-conjugated polymer enable this polymer to become an efficient carrier for drugs.
Acknowledgement
M.H.S. thanks the Australian Research Council (ARC) for funding.