Physically and Chemically Cross-Linked Poly{[(maleic anhydride)-alt-styrene]-co-(2-acrylamido-2-methyl-1-propanesulfonic acid)}/Poly(ethylene glycol) Proton-Exchange Membranes
Yılser G. Devrim
Department of Chemical Engineering, Faculty of Engineering, Hacettepe University, Beytepe, 06800 Ankara, Turkey
Search for more papers by this authorZakir Rzaev
Department of Chemical Engineering, Faculty of Engineering, Hacettepe University, Beytepe, 06800 Ankara, Turkey
Search for more papers by this authorCorresponding Author
Erhan Pişkin
Department of Chemical Engineering, Faculty of Engineering, Hacettepe University, Beytepe, 06800 Ankara, Turkey
Department of Chemical Engineering, Faculty of Engineering, Hacettepe University, Beytepe, 06800 Ankara, Turkey. Fax: +90 312 299 2124Search for more papers by this authorYılser G. Devrim
Department of Chemical Engineering, Faculty of Engineering, Hacettepe University, Beytepe, 06800 Ankara, Turkey
Search for more papers by this authorZakir Rzaev
Department of Chemical Engineering, Faculty of Engineering, Hacettepe University, Beytepe, 06800 Ankara, Turkey
Search for more papers by this authorCorresponding Author
Erhan Pişkin
Department of Chemical Engineering, Faculty of Engineering, Hacettepe University, Beytepe, 06800 Ankara, Turkey
Department of Chemical Engineering, Faculty of Engineering, Hacettepe University, Beytepe, 06800 Ankara, Turkey. Fax: +90 312 299 2124Search for more papers by this authorAbstract
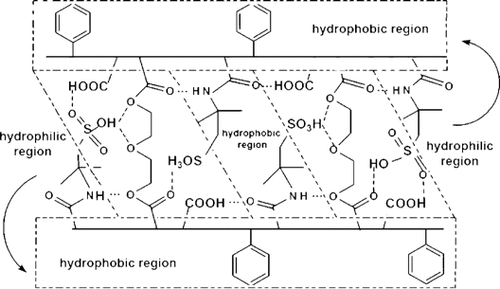
Novel proton exchange membranes were solvent-cast from DMF solutions of the terpolymers poly[(MA-alt-S)-co-AMPS], containing hydrophobic phenyl and reactive hydrophilic carboxylic and organo-sulfonic acid fragments with different compositions, and PEGs with different molecular weights and amounts. These membranes were formed as a result of physical (via H-bonding) and chemical (via PEG) cross-linking. The structures of membranes were confirmed by FT-IR and 1H- and 13C NMR spectroscopy. Mechanical and thermal properties, swellability, and proton conductivity of these membranes were significantly affected both by the chemical composition of the terpolymers (mainly the AMPS content) and also the cross-linker (PEG) molecular weight and content in the final form of the membranes. It was concluded that the membranes prepared by using the terpolymer with an AMPS content of 36.84 mol-% and PEG with a molecular weight of 1 450 and with an initial PEG content of 30 wt.-% are the most suitable ones for fuel cell applications.
References
- 1 D. Linden, “ Handbook of Batteries and Fuel Cells”, McGraw-Hill, New York 1984.
- 2
K. Kordesch,
G. Simader,
“ Fuel Cells and Their Applications”,
VCH,
Weinheim
1996.
10.1002/352760653X Google Scholar
- 3 T. V. Nguyen, N. Vanderborgh, J. Membr. Sci. 1998, 143, 235.
- 4
W. S. Ho,
K. K. Sirkar,
“ Membrane Handbook”,
Van Nostrand Reinhold,
New York
1992.
10.1007/978-1-4615-3548-5 Google Scholar
- 5 P. L. Antonucci, A. S. Arico', P. Criti, E. Ramunni, V. Antonucci, Solid State Ionics 1999, 125, 431.
- 6 P. Costamagna, C. Yang, A. B. Bocarsly, S. Srinivasan, Electrochim. Acta 2002, 4, 1023.
- 7 M. A. Hickner, H. Ghassemi, Y. S. Kim, B. R. Einsla, J. E. McGrath, Chem. Rev. 2004, 104, 4587.
- 8 P. D. Beattie, F. P. Orfino, V. I. Basura, K. Zychowska, J. F. Ding, C. Chuy, J. Schmeisser, S. Holdcroft, J. Electroanalyt. Chem. 2001, 503, 45.
- 9 J. Larminie, A. Dicks, “ Fuel Cell Systems Explained”, J. Wiley & Sons, New York 2000.
- 10 H. S. Mansur, R. L. Oréfice, A. A. P. Mansur, Polymer 2004, 45, 7193.
- 11 S. D. Mikhailenko, K. Wang, S. Kaliaguine, P. Xing, G. P. Robertson, M. D. Guiver, J. Membr. Sci. 2004, 233, 93.
- 12 M. S. Kang, Y. J. Choi, S. H. Moon, J. Membr. Sci. 2002, 207, 157.
- 13 M. S. Kang, S. H. Cho, H. S. Kim, Y. J. Choi, S. H. Moon, J. Membr. Sci. 2003, 222, 149.
- 14 M. S. Kang, Y. J. Coi, S. H. Moon, J. Membr. Sci. 2002, 207, 157.
- 15 J. H. Kim, J. M.-S. Kang, C. K. Kim, J. Membr. Sci. 2005, 265, 167.
- 16 B. Smitha, S. Sridhar, A. A. Khan, Macromolecules 2004, 37, 2233.
- 17 J. H. Kim, B. R. Min, K. B. Lee, J. Won, Y. S. Kang, Chem. Commun. 2002, 2732.
- 18 D. S. Kim, H. B. Park, J.-W. Rhim, Y. M. Lee, J. Membr. Sci. 2004, 240, 37.
- 19
B. C. Trivedi,
B. M. Culberton,
“ Maleic Anhydride”,
Plenum,
New York
1982.
10.1007/978-1-4757-0940-7 Google Scholar
- 20 Z. M. O. Rzaev, “ Polymers and Copolymers of Maleic Anhydride”, Elm, Baku 1984.
- 21 J. M. G. Cowie, “ Alternative Copolymers”, Plenum, New York 1985.
- 22 C. Vanclair, P. Schaetzel, R. Nobrega, C. Habert, J. Appl. Polym. Sci. 2002, 86, 1709.
- 23 L. Ya. Tsarik, G. P. Mantsivoda, G. V. Ratovskii, Vysokomol. Soedin. 1977, A19, 601.
- 24 O. Savadgo, J. New Mater. Electrochem. Syst. 1998, 1, 47.
- 25 Z. Florjanczyk, E. Zygaldo-Monikowska, E. Wielgus-Barry, K. Kuzwa, J. Pasniewski, Electrochim. Acta 2003, 48, 2201.
- 26 H. Erdemi, A. Bozkurt, W. H. Meyer, Synth. Met. 2004, 143, 133.
- 27 C. W. Walker, J. Power Sources 2002, 110, 144.
- 28 C. Zhang, A. J. Easeal, J. Appl. Polym. Sci. 2004, 91, 3635.
- 29 C. Zhang, A. J. Easeal, J. Appl. Polym. Sci. 2004, 94, 2083.
- 30 L. E. Karlsson, B. Wesslen, P. Jannasch, Electrochim. Acta 2002, 47, 3269.
- 31 US 3929741 (1975), Datascore Co., inv.: R. A. Laskey.
- 32 J. Travas-Sejdic, A. J. Easteal, Polym. Gels Networks 1997, 5, 481.
- 33 Y. Liu, J.-J. Xie, X.-Y. Zhang, J. Appl. Polym. Sci. 2003, 90, 481.
- 34 T. Yamakawa, S. Ishida, M. Higa, K. Matsusaki, Trans. Mater. Res. Soc. 2003, 28, 829.
- 35 M. Higa, T. Yamakawa, J. Phys. Chem. B 2004, 108, 16703.
- 36 T. Yamakawa, S. Ishida, M. Higa, J. Membr. Sci. 2005, 250, 61.
- 37 S. J. Yang, W. Jang, C. Lee, Y. G. Shul, H. Han, J. Polym. Sci., Part B: Polym. Phys. 2005, 43, 1455.
- 38 Y. G. Devrim, Z. M. O. Rzaev, E. Pişkin, 40th IUPAC World Polymer Congress, Paris, 2004, http://www.e-polymers.org/paris.
- 39 Y. Devrim, Z. M. O. Rzaev, E. Pişkin, European Polymer Congress, Moscow, 2005, Section 6.1.5, Abstract R2058.
- 40 Y. Devrim, Z. M. O. Rzaev, E. Pişkin, Macromol. Chem. Phys. 2006, 207, 111.
- 41 B. C. Kim, G. M. Spinks, C. O. Too, G. G. Wallace, Y. H. Bae, N. Ogata, React. Funct. Polym. 2000, 44, 245.
- 42 S. L. Chen, L. Krishnan, S. Srinivasan, J. Benziger, A. B. Bocarsly, J. Membr. Sci. 2004, 243, 327.
- 43 J. F. Rabek, “ Experimental Methods in Polymer Chemistry”, Wiley, New York 1980.
- 44 J. W. Rhim, H. B. Park, C. S. Lee, J. H. Jun, D. S. Kim, Y. M. Lee, J. Membr. Sci. 2004, 238, 143.
- 45 A. M. Elmer, P. Jannasch, Polymer 2005, 46, 7896.
- 46 H. Pu, L. Qiao, Macromol. Chem. Phys. 2005, 206, 263.
- 47 A. F. Ismail, N. Zubir, M. M. Nasef, K. M. Dahlan, A. R. Hassan, J. Membr. Sci. 2005, 254, 189.