Long-term outcome of primary percutaneous stent angioplasty for pediatric posttransplantation portal vein stenosis
Abstract
This study aims to evaluate the long-term efficacy and reintervention rate after primary percutaneous portal vein stent angioplasty for portal vein stenosis (PVS) in pediatric liver transplantation (LT) recipients. From 2004 to 2020, a total of 470 pediatric LTs were performed in our center. All cases were screened for interventional PVS treatment and analyzed retrospectively. We identified 44 patients with 46 percutaneous angioplasties for posttransplantation PVS. The median interval from LT to percutaneous catheter intervention was 5 months (16 days–104 months) with a median follow-up (f/u) period after catheter intervention of 5.7 years (2–156 months). In 40 patients, an endovascular stent was placed as primary (n = 38) or secondary (n = 2) intervention. The median age at stent placement was 23 (6–179) months with a median weight of 10 kg (6–46 kg). Technical success and relief of PVS were achieved in all patients irrespective of age or weight. Adverse events occurred peri-interventionally in two patients and were resolved with standard care. All primary portal vein (PV) stents remained patent until the end of f/u. Reinterventions have been successfully performed in 10 patients for suspected or proven restenosis, resulting in a primary patency rate of 75% and an assisted patency rate of 25%. The median time to reintervention was 6.2 years (range 1–10 years). The need for reintervention was independent of age or weight at both transplantation and initial angioplasty as well as of additional risk factors due to portal hypertension. Percutaneous transhepatic PV stent angioplasty in children is safe and effective in all age groups, with excellent long-term patency. Primary stent angioplasty should be considered as first-line treatment for PVS after pediatric LT.
Abbreviations
-
- BA
-
- biliary atresia
-
- DDLT
-
- deceased donor liver transplantation
-
- f/u
-
- follow-up
-
- GRWR
-
- graft-to-recipient weight ratio
-
- Left lateral
-
- liver segments II + III
-
- LDLT
-
- living donor liver transplantation
-
- LT
-
- liver transplantation
-
- NA
-
- not available
-
- PTA
-
- percutaneous angioplasty
-
- PTBA
-
- percutaneous transluminal balloon angioplasty
-
- PTSA
-
- percutaneous transluminal stent angioplasty
-
- PV
-
- portal vein
-
- PVC
-
- portal vein complication
-
- PVS
-
- portal vein stenosis
-
- PVT
-
- portal vein thrombosis
-
- US
-
- ultrasound
-
- vmax
-
- maximum flow velocity (Doppler US)
INTRODUCTION
Pediatric liver transplantation (LT) is a well-established treatment for end-stage liver disease.[1] Split LT has been introduced to increase organ availability for children. The technique is technically demanding because of short vascular pedicles and size mismatches, contributing to vascular complications.
Posttransplantation portal vein complications (PVCs) occur in 2%–14% of pediatric patients with LT[2-4] and consist of portal vein (PV) thrombosis (PVT) or PV stenosis (PVS).[5, 6]
PVS has a prevalence of 3%–8%[2, 7-9] and may lead to portal hypertension and subsequent graft failure, necessitating retransplantation. Early diagnosis and adequate treatment of PVS are crucial. Besides surgical revision, percutaneous intervention has emerged as the primary treatment for posttransplantation PVS. Percutaneous transluminal balloon angioplasty (PTBA) and stent placement (percutaneous transluminal stent angioplasty [PTSA]) are less invasive and more effective compared with surgery.[10-12] Stent angioplasty has been performed by most groups only when balloon angioplasty failed or long-term patency could not be achieved.[8, 11, 13] Our center favored stent placement as primary treatment option for 15 years. This study aims to evaluate the efficacy and long-term outcome of percutaneous PV stent angioplasty after pediatric LT as primary therapy.
PATIENTS AND METHODS
This study was conducted retrospectively at the Hanover Medical School Children's Hospital, one of the largest pediatric LT centers in Europe. Based on the hospital's electronic health records system, we reviewed all medical records of patients who underwent pediatric LT between 2004 and 2020 and identified all cases with percutaneous intervention. Analyses were conducted in accordance with the local institutional review board (ethics committee, Hannover Medical School, N 9804_BO_K_2021).
PV reconstruction during LT
The type of graft (whole, reduced size, split) as well as the individual recipient anatomy defined how reconstruction of the PV was performed. In general, in grafts with regular hilar region for whole and reduced size organs, PV anastomosis was performed in an end-to-end fashion. For splits, the PV usually is reconstructed by an anastomosis between the left PV of the donor and a right-/left-branch patch of the recipient PV in order to overcome the usually observed difference in diameter between adult and pediatric vessels. In case of fibrotic transformation of the recipient PV (as often seen in biliary atresia), the donor vein inserted directly at the confluence of the vessel is long enough or the recipient PV is replaced by a size-adapted allograft. To optimize PV flow, any known or intraoperatively identified collaterals are ligated as reported by others.[14] Left gastric vein was ligated in case of insufficient flow; likewise, interruption of the left renal vein was attempted to improve portal flow in selected cases.
Definition of PVS and intervention criteria
- clinical symptoms of portal hypertension: ascites, splenomegaly, gastrointestinal tract bleeding from varices, and thrombocytopenia; or
- US findings: PV diameter <2.5 mm, pre/poststenotic dilatation, maximum trans-stenotic flow velocity (vmax) acceleration, turbulence, diminished PV flow (<10 cm/s), no flow, or retrograde PV perfusion, and presence of collaterals.
Angiographic evaluation confirmed the diagnosis of PVS and assessed the hemodynamic relevance, including pre- and postinterventional trans-stenotic pressure gradient and collateral flow in the extrahepatic PV circulation. If applicable, stenosis of >50% prestenotic PV diameter and/or a trans-stenotic pressure gradient of ≥3.0 mm Hg served as criteria for intervention.
Procedural details
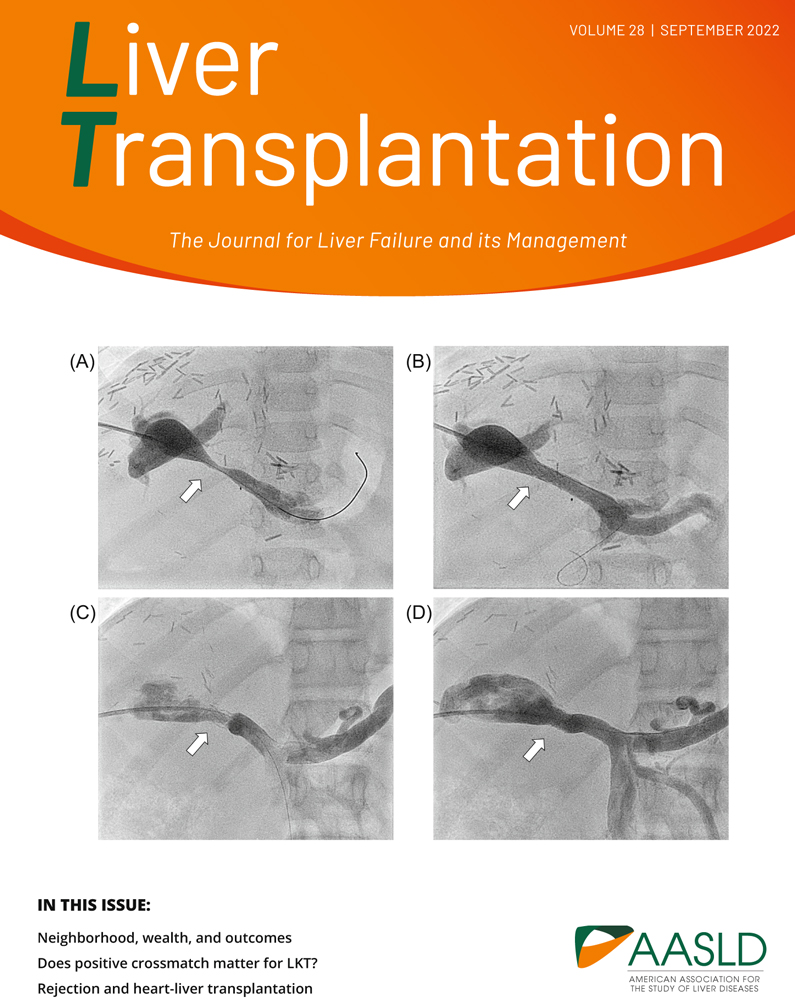
Percutaneous interventions were performed under general anesthesia. We established transhepatic access to the intrahepatic PV in most patients via intercostal puncture using micropuncture sets (Cook Medical, Bloomington, NJ) with a standard 21G needle under US and fluoroscopic guidance. A 0.018″ soft-tip wire or a coronary wire was used to pass through the stenotic vascular segment followed by 4.0- or 5.0-F diagnostic catheters for pressure monitoring and selective angiography. Transhepatic (n = 32) or trans-splenic (n = 8) PV angioplasties were performed with premounted stents (Palmaz Blue; Cordis Corporation, Warren, NJ; n = 25) in infants and small children, with a balloon diameter of 5–7 mm and length of 12–18 mm. Palmaz Genesis (Cordis Corporation, Miami, FL; n = 11) and VALEO stent (Bard Inc., New Providence, NJ; n = 1) were reserved for children with larger PV diameter or reinterventions. The balloon size was chosen according to the prestenotic PV diameter of either the portal or mesenteric vein. Distinct stent oversizing was avoided for the prevention of endothelial damage with the risk of PVT. Portography and manometry were repeated to evaluate procedural effectiveness and exclude vessel injury (Figure 1).
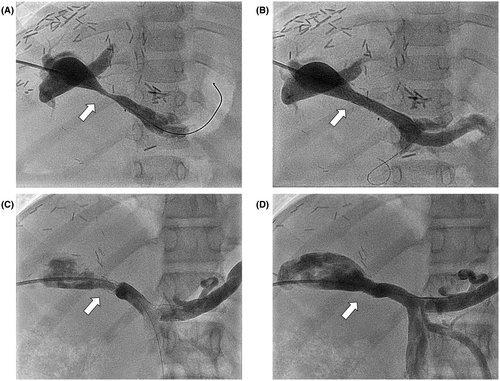
The trans-splenic approach has been described in detail elsewhere.[15] It was used for patients with complete PVT, failed transhepatic approach, or increased risk for transhepatic puncture. Neither intraoperative PV stenting during LT[16] nor direct access to the PV via minilaparotomy[17, 18] were performed during the study period in our center.
After successful sheath introduction, the patients received heparin 50–100 IE/kg as bolus injection followed by 400 IE/kg/day for 24–48 h after catheterization. A single dose of antibiotic was administered to every patient with stent angioplasty. Unless contraindicated by increased bleeding risks or thrombocyte count below 50,000/μl, a platelet inhibitor (acetylsalicylic acid 1–3 mg/kg/day or clopidogrel 0.5–2 mg/kg/day) was added for at least 3–6 months. US was performed at the end of the procedure and at least twice within the first 48 h after the intervention to rule out bleeding complications.
Technical success, clinical success, and complications
- stent placement at the intended location,
- no or insignificant residual stenosis (increase of stenotic diameter >150%),
- no or minimal residual pressure gradient (≤2.0 mm Hg), and
- improved intrahepatic PV flow and reduced collateral flow postinterventionally.
Clinical success was defined as improved portal hypertension (e.g., reduction of ascites, splenomegaly, gastrointestinal tract bleeding, and thrombocytopenia).
Adverse events were graded according to the guidelines of the Cardiovascular and Interventional Radiological Society of Europe[19] and the Society of Vascular Surgery.[20] The need for additional surgery, unplanned catheter intervention, adverse sequelae, and death were considered severe complications. Other events were considered mild or moderate events.
Follow-up, patency, and risk factors for postinterventional restenosis
Clinical, laboratory, and imaging parameters were monitored during standardized follow-up (f/u) visits according to the local pediatric LT protocol. For this study, postinterventional in-hospital examinations and f/u visits 2 weeks; 3–6 months; and 1, 3, 5, and 10 years after PTBA were analyzed. During f/u visits, PV stent diameter and flow velocity were routinely evaluated by Doppler US to assess stent patency. According to the criteria of the Society of Vascular Surgery,[20] primary patency was defined as no need for additional endovascular intervention after placement. Assisted patency was considered as status after reintervention during f/u. PV status after total occlusion followed by reintervention was classified as secondary patency.[18] Patients with primary PTBA and subsequent stent placement for restenosis were considered patients with PTSA during further f/u. Retransplantation was considered the end of f/u period.
There are risk factors for development of posttransplantation PVS reported in the literature: age, weight, underlying disease, and surgical details; see Table 1. We initially hypothesized that these parameters as well as early posttransplantation PVS would result in a higher risk of restenosis after stent angioplasty and potentially lead to a shorter primary patency. We used the Cox regression model to analyze the effect of those factors on the freedom of reintervention and divided patients into subgroups below or above (1) 8-kg bodyweight or (2) 12 months of age at LT, (3) 10-kg body weight or (4) 24 months of age at PTSA, and (5) time between LT and PTSA below or above 3 months. Next, we assumed that suffering from fixed portal hypertension (chronic end-stage liver disease) might also play a role in a higher restenosis rate. Therefore, we introduced an additional risk profile based on the following criteria: prior abdominal surgery (e.g., Kasai operation), liver retransplantation, PVT followed by surgical revision, PV collaterals, and retrograde PV flow. Each patient's risk for PV restenosis was assessed using a risk score, resulting in three groups with low, moderate, or high risk.
| Author/year | Significant risk factor for PVC | p value | Cohort | PVC |
|---|---|---|---|---|
| Ueda et al.[2] 1990–2003 | Low body weight <6 kg | 0.035 | 527 pediatric LDLTs (left lateral) |
Early: 9 (1.7%) Late: 39 (8.0%) |
| Sieders et al.[36] 1982–1999 | Young age 1.1 (years; range 0.2–3.3) | <0.01 | 157 pediatric LTs | 7 (4%) PVTs |
| Low body weight 8.5 kg (6.4–14.4) | <0.05 | |||
| Left lateral segment | 0.03 | |||
| Chardot et al.[37] 1988–1995 | PV diameter 3.5 ± 1.32 mm | 0.006 | 180 pediatric LTs in 156 patients; 115 pediatric LTs in 96 patients with BA |
BA: 19 (16.5%; 16 PVTs + 3 PVS) Non-BA: 3 (4.6%) PVTs |
| Young age 1.76 ± 0.77 years | 0.018 | |||
| Low body weight 10.42 ± 2.32 kg | 0.031 | |||
| Vasavada et al.[38] 2003–2013 | Young age 11.5 vs. 14.5 months | 0.024 | 110 pediatric LDLT (BA) | 10 (9.1%; 5 PVTs +5 PVSs) |
| Lower weight 7.65 vs. 9 kg | 0.022 | |||
| Higher GRWR 3.51 vs. 3.07 | 0.018 | |||
| Moon et al.[39] 1997–2008 | PV diameter <5 mm | 0.012 | 96 pediatric LDLT | 11 (11.5%) |
| Body weight <8 kg | 0.193 | |||
| Lucianetti et al.[40] 1997–2004 | Body weight <6 kg | NA | 18 pediatric LTs <6 kg (15 left lateral) | 1 PVT (6%) |
- Note: Table 1 summarizes the literature on risk factors for PV complications after pediatric LT. All authors identified low body weight at transplantation (bold). However, most studies suffer from selection bias, low patient numbers, or both. Ueda et al.[2] report a cohort with a comparable number of patients as reported in this study. Of note, their cohort only contained left lateral split transplantation, whereas our cohort includes all organ types.
- Abbreviations: BA, biliary atresia; GRWR, graft-to-recipient weight ratio; LDLT, living donor liver transplantation; left lateral, liver segments II + III; LT, liver transplantation; NA, not available; PV, portal vein; PVC, portal vein complication; PVS, portal vein stenosis; PVT, portal vein thrombosis.
Statistical analysis
Statistical analysis and visual representation of data were performed using the statistical programming language R (https://www.r-project.org/) version 1.3.1093. Parametric and nonparametric univariate and multivariate statistical testing were conducted for the analysis of the data. Student t test was used for normal distributed data. Other data were analyzed using Wilcoxon signed rank test. Cox regression and Kaplan–Meier analysis were used to analyze freedom from reintervention survival, with log-rank testing to discriminate between subgroups. Statistical significance was defined at a p value of <0.01.
RESULTS
Cohort characterization
Between 2004 and 2020, 470 pediatric LTs (median 2.0 years) have been carried out at our center. PVS was found in 44 patients (9.3%) receiving a total of 46 PV interventions.
Forty patients received a PV stent and were analyzed retrospectively in this study. In most patients (n = 38), stent angioplasty was performed as primary intervention. In two out of six patients, it was performed as second percutaneous intervention after previous balloon angioplasty.
The median time interval from LT to PTSA was 5.6 months, resulting in median age and weight at the percutaneous intervention of 23.5 months and 10 kg, respectively. For a detailed cohort description, see Tables 2 and 3.
| 2004–2020 | LT | PVS |
|---|---|---|
| n | 470 | 44 |
| Median age, months | 24 (1–252) | 10 (5–178) |
| Whole | 139 | 5 |
| Left lateral | 248 | 37 |
| LDLT | 96 | 14 |
| Biliary atresia | 178 | 31 |
- Note: Characteristics of 470 pediatric LTs between 2004 and 2020 screened for PVS. For both groups, the mean age and graft type are stated. Biliary atresia is shown as the most common underlying disease in patients with PVS.
- Abbreviations: LDLT, living donor liver transplantation; left lateral, liver segments II + III; LT, liver transplantation; PVS, portal vein stenosis; whole, whole liver graft.
| PTA (all) | PTSA | |
|---|---|---|
| Sex | ||
| Male | 26 (57) | 17 (42.5) |
| Female | 20 (43) | 23 (57.5) |
| Donor | ||
| DDLT | 32 (70) | 29 (72.5) |
| LDLT | 14 (30) | 11 (27.5) |
| Organ type | ||
| Whole | 5 (11) | 4 (10) |
| Left lateral | 36 (78) | 34 (85) |
| Right | 3 (7) | 2 (5) |
| Anastomosis | ||
| End to end | 31 (67) | 27 (67.5) |
| Branch patch | 6 (13) | 5 (12.5) |
| Vein graft | 5 (11) | 5 (12.5) |
| Jump graft | 3 (75) | 2 (5) |
| Weight at LT, kg | 8 (5–61) | 8 (5–21) |
| Age at LT, months | 10 (5–178) | 9.5 (5–98) |
| Time to intervention, months | 5 (0.4–104) | 5.6 (0.5–104) |
| Weight at intervention, kg | 11 (6–52) | 10 (6–46) |
| Age at intervention, months | 26 (10–180) | 23.5 (6–179) |
| Freedom of reintervention, months | 69; 69; 2–156 | 70; 69; 2–156 |
- Note: Data are presented as n (%), median (range), or mean; median; range.
- Abbreviations: DDLT, deceased donor liver transplantation; LDLT, living donor liver transplantation; left lateral, liver segments II + III; LT, liver transplantation; PTA, percutaneous transluminal angioplasty; PTSA, percutaneous transluminal stent angioplasty; PV, portal vein.
All patients with PTSA showed relevant laboratory pathology (thrombopenia, mild increase of hepatic transaminases without other etiology such as graft rejection) and aggravating US findings during f/u, such as smaller PV diameter, progressive splenomegaly, increasing collateral flow, and reduced antegrade PV flow with risk for subsequent thrombosis.
Ascites was the leading symptom of relevant PVS in the subacute phase after LT. Twelve patients required continuous drainage and permanent intravenous fluid substitution before catheter intervention. By contrast, ascites was documented in only two patients with PVS occurring later than 6 months after LT.
Percutaneous intervention (technical success, clinical success, complications)
In 34 patients, the intrahepatic PV could successfully be reached through transhepatic access (Figure 1). In a single patient, this was switched to trans-splenic puncture during the same intervention. Trans-splenic access, as described before,[15] was the primary access route in five patients.
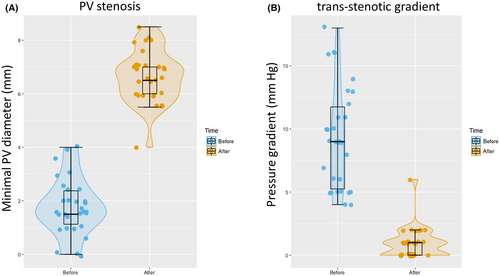
Technical success could be achieved in all patients. Stent angioplasty led to a significant increase in the mean stenotic diameter (1.7–6.4 mm) and a significant reduction in the mean trans-stenotic pressure gradient (9.3–0.9 mm Hg residual gradient; Figures 1 and 2). In 10 cases, data before intervention were not available due to (sub)total occlusion of the vessel.
Six patients (median 26 months) in our cohort received primary PTBA. In this small group, the mean PV diameter could be increased by 3 mm (4–7 mm), reducing the mean PV gradient from 5.0 to 1 mm Hg. According to PV pressure and minimal PV diameter, these patients may retrospectively be considered having moderate PVS at the time of first intervention, except one with PVT, as described elsewhere.[15] One case turned out as kinking PV with only mild PVS (diameter 7 mm, gradient 2 mm Hg) with almost no change after intervention (diameter 8 mm, gradient 2 mm Hg).
All patients, except for one patient with subarachnoidal bleeding, received standard anticoagulation with heparin as described in procedural details. Antiplatelet therapy with acetylsalicylic acid, clopidogrel, or both were given for at least 3–6 months. The duration of treatment was decided on the individual risk assessment for PVT.
The standard immune suppression regimen in our LT center is tacrolimus. PVS intervention alone did not lead to a change in immunosuppression.
In two patients, in-stent thrombosis or perisplenic hematoma occurred independently as complications of PTSA. Both conditions resolved with conservative treatment alone and were therefore graded as moderate/grade 3.[19, 20] Early in-stent thrombosis occurred in coincidence with peri-interventional infection and resulted in a residual gradient of 6.0 mm Hg. After successful treatment with high-dose intravenous heparin (600 IU/kg/d) over 96 h in combination with platelet inhibition and intravenous antibiotics, patency was achieved for currently more than 12 years. Puncture-related perisplenic hematoma was not accompanied by hemodynamic compromise or a significant drop in hemoglobin concentration, and no vessel damage was found. The patient received a single elective transfusion of red cell concentrate.
A reduction of the size of the spleen and an increase in platelet count are major components of postinterventional clinical success. Splenomegaly of 3.4 cm above 95th percentile (average, long axis) was found in 37/38 patients before intervention. Two patients were excluded from this statistic due to polysplenia. Within the first 6 months after PTSA, spleen size decreased by an average of 1.5 cm in 30/38 patients. Half of the patients (10/20) reached a normal diameter at the 5-year f/u. A reduction in diameter persisted in 18/20 patients for >5 years and 7/8 patients for >10 years. Concordantly, the mean platelet count increased significantly (+20%) in 26/39 patients within 1 year (187,000/μl vs. 236,000 tsd/μl; p < 0.01).
The mean PV maximum flow velocity (vmax) before intervention was 160 cm/s (median 180 cm/s; maximum 400 cm/s; data available from 36 patients). Successful PTSA led to a significant decrease of >50% in PV flow velocity 1 year after intervention.
Collaterals due to portal hypertension could be identified in 16 patients before catheter intervention. In this group, PTSA resulted in a decrease of the mean PV flow in nine patients, whereas the vmax increased (55 to 89 cm/s) in seven patients. Accompanying PVT was found in five patients during angiography (4/5) but did not affect interventional success.
Gastrointestinal bleeding was seen in five patients with coexisting portosystemic shunts before percutaneous angioplasty (PTA) and did not occur after treatment.
In all 12 patients, with free abdominal fluid due to early postoperative PVS, stent angioplasty resolved ascites, allowing subsequent removal of the drainage, recovery, and discharge.
Follow-up, patency, and risk factors for postinterventional restenosis
Thirty-eight patients with PTSA had a f/u of at least 1 year, 21 patients >5 years, and eight patients ≥10 years. The median clinical f/u period was 5.7 years (range 2–156 months). The maximum f/u period was 13 years.
One patient underwent retransplantation during f/u due to biliary complications, without evidence for restenosis at the time of reoperation. Intraoperatively, the stent could be removed without interference with the new graft. One patient died of pneumonia 1 year after intervention.
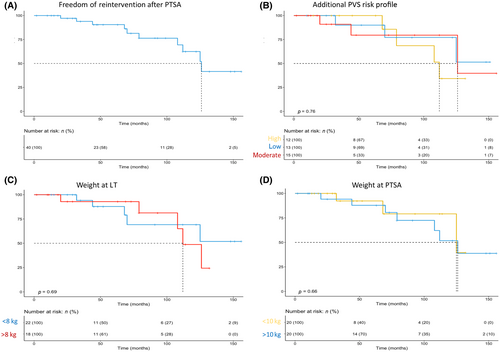
Because of the variance in f/u length, primary patency rates were calculated at standardized time points with patients' adequate f/u data. We report an overall primary patency rate of 75% with rates of 100%, 90%, 91%, 77%, and 75% after 1, 3, 5, 7, and 10 years, respectively. For detailed patency rates, see Figure 3A.
Two primary patients with PTBA received secondary stents for recurrent PVS 8 and 15 months after initial angioplasty, respectively.
Ten patients received diagnostic catheterization for suspected in-stent stenosis during f/u; for detailed information on patients with restenosis, see Tables S2 and S3. Time to reintervention ranged from 20 to 126 months (mean/median 78.4/74.5 months) after the first PTSA. Restenosis was only evident in 5 of these 10 patients, resulting in reangioplasty. In two patients, a second stent was telescoped into the first one, and three patients could be treated with balloon angioplasty alone (Figure 1). The other five patients showed neither a reduction in PV diameter nor a pressure gradient but received prophylactic balloon angioplasty to adapt the stent diameter to somatic growth. As interventional catheterization was performed, all 10 patients were classified as assisted patency, independent of objective restenosis before reintervention.
All reinterventions could be carried out successfully without any complication, resulting in an overall assisted patency rate of 100%.
Besides the risk factors of young age, low body weight, and early posttransplantation PVS, all patients were systematically analyzed for the preexisting additional risk factors linked to fixed portal hypertension. Our cohort showed an almost even distribution with 13, 15, and 12 patients at low, moderate, and high risk, respectively. Most patients at low risk had received LT for acute liver failure without symptoms of chronic portal hypertension such as reverse portal flow or extensive collaterals. However, neither in the univariate nor in the multivariate Cox regression analysis did any of these parameters show a significant effect on primary patency (Figures 3 and S1, Table S4).
Reinterventions were performed in 3/27 patients with end-to-end anastomosis and 7/13 patients with atypical anastomosis; see Table S2. Although indicating a higher restenosis rate in patients with atypical PV anastomosis, low case numbers prevented statistical comparison of different types of PV anastomosis.
DISCUSSION
PVS after pediatric LT is a frequent complication and observed during long-term f/u with a rate of 3%–8%[2, 7-9] and may lead to portal hypertension and subsequent graft failure, necessitating retransplantation.
Percutaneous PV intervention in children remains an invasive procedure with technically demanding vascular access requiring general anesthesia. Previous studies have reported the effectiveness and safety of interventional treatment on PVS in adults[21] and children.[8, 10, 22, 23] As a result of the high technical and clinical success rates between 76% and 100%, most patients with PVS are nowadays referred to interventional treatment.[8, 11, 23-25]
Controversy remains as to whether balloon or stent angioplasty should be the primary treatment option for PVS after pediatric LT. Most groups favored PTBA over primary stent implantation, reserving stent angioplasty only for failing balloon dilatation, early interventions in the subacute phase after LT, and special conditions such as graft interposition. However, this reservation was partly due to some technical limitations of the early days of posttransplantation intervention. Most interventional radiologists used a sheath of at least 7F for percutaneous interventions. The long self-expanding wall stents covered long segments of the intra- and extrahepatic PV and parts of the mesenteric or splenic vein. Traditionally, there have also been reservations against PV stenting by some transplant surgeons due to potential technical problems in case of a retransplantation.[9-13, 26]
In our opinion, every intervention should strive to achieve the longest patency and the lowest reintervention rate possible. Therefore, our preferred strategy for posttransplantation PVS has been primary PTSA with balloon-expandable stents according to the nonaffected vessel's size for >15 years. The balloon-expandable stents used in this study were 12–18 mm short and enabled a 5F sheath in all infants and small children, whereas larger sheaths were reserved for adolescents. In the case of recurrent PVS, the size of the stent might be adjusted with larger balloons. Alternatively, a larger second stent might be implanted within the first one, followed by redilatation of both stents with high-pressure balloons.
Technical and clinical success, complications of primary PTSA
The most reliable parameters for assessing the immediate effectiveness of angioplasty include the trans-stenotic pressure gradient and the diameter of the stenotic segment. In our cohort, both parameters improved significantly after PTSA in every patient securing markedly improved intrahepatic PV perfusion after intervention (Figure 2A).
Long-term success was evaluated based on clinical criteria such as platelet count, splenomegaly, PV flow, and portosystemic shunts. Comparable with the excellent hemodynamic success achieved, we report a significant clinical improvement after percutaneous PV intervention. Within the first months after PTSA, we noted a highly significant (p < 0.01) increase in the mean platelet count, comparable to clinical success data reported by Cheng et al. in a similar cohort (n = 11; 79,000 vs. 135,000/μl).[27] At the same time, the diameter of the spleen decreased significantly by an average of 1.5 cm (p < 0.01). In our cohort, ascites was present in approximately one-third of the patients (12/40, predominantly in the subacute phase after LT) and gastrointestinal bleeding occurred in five children before intervention (11%), with two patients receiving blood transfusions. Neither of these symptoms recur postinterventionally.
Cheng et al. reported promising results after primary PTSA in a small cohort with 4 adult and 12 pediatric patients (mean age/weight 5.8 years/21 kg). The reported technical success was 68.8%.[28] Ko et al.[29] showed even better results in a small pediatric cohort (n = 12). Other authors reported a comparable reduction of trans-stenotic pressure gradient.[8, 10-13, 30] However, most of these studies reported combined balloon and stent angioplasty data and PV diameters are often not reported. In a recently published systemic review of the literature, Sare et al.[31] concluded that primary PTSA had technical success of 100%, which was significantly higher compared with balloon angioplasty. They clearly favored primary stent implantation as treatment of choice.
In two patients, moderate complications occurred during percutaneous intervention and resolved with standard conservative care. This reflects the safety of primary stent placement in an experienced pediatric catheterization laboratory, even in infancy. Other groups report comparable results.[10, 14, 27]
Patency rate, reintervention, and risk for postinterventional restenosis
All PV stents remained patent until the last f/u visit. With 10/10 successful reinterventions (for detailed information, see Tables S2 and S3), we report an overall primary patency rate of 75% and an assisted patency of 100%. Because of the variance in f/u length, primary patency was calculated at standardized time points of 1, 3, 5, 7, and 10 years resulting in 100%, 90%, 91%, 77%, and 75%, respectively. In five patients, PV stents did not need any reintervention for >10 years (maximum 13 years). In the literature, patency rates of primary PTSA have been reported to be 90.9% (f/u 12 months)[27] or 100% (f/u 10–58 months).[29] This is in marked contrast to PTBA cohorts, where comparable patency could only be achieved with recurrent reinterventions with up to five additional balloon dilatations[11] or secondary stent placement,[26] respectively.
Early data by Funaki et al. in 1997 showed a 64% recurrence rate of PVS in 11 pediatric patients within 1–13 months (mean 6.3 months) after PTBA. Stent placement could achieve longer freedom from reintervention.[13, 23, 24] More recent reports by Yabuta et al.[11] and Patel et al.[8] favoring primary PTBA showed improved early results with lower reintervention rates: 11/43 patients (mean age at intervention 4.1 years)[11] and 13/41 (median age 1.7 years),[8] respectively. However, in the first pediatric cohort, eleven patients underwent two, six patients needed three, and two received five interventions until patency was achieved.[11] In the second group of smaller patients, the authors stated that just over half of their patients eventually required stent placement during f/u.[26]
Based on these results from the literature, balloon angioplasty of PVS is generally burdened by high recurrence rates with the need for one or more reinterventions in <2 years of f/u. Shim et al. reported no significant difference in long-term outcome between both techniques.[32] However, although their PTSA primary patency rate was 100%, three patients with balloon angioplasty needed reintervention. In our opinion, this reflects one of the key points of percutaneous intervention for PVS. Although technical success may be achieved regardless of the technique, balloon angioplasty has higher reintervention rates, making stent angioplasty the superior technique. This is in line with a recent systematic review of 243 pediatric patients: primary PTSA has higher technical success and lower restenosis rates (2.5% vs. 29.7%) compared with PTBA alone.[31]
Notably, in our cohort, almost half of the patients (19/40) successfully received PTSA within 6 months after LT and 6 within 45 days after LT (PTSA n = 4; PTBA n = 2). PVS early after LT is often more elastic, and PTBA alone might be ineffective and more prone to a possible vessel tear at the anastomotic site. This is why in these patients, primary PTSA is recommended even in the subacute phase after transplantation to avoid early reoperation.[29] Although both balloon and stent angioplasty are considered safe and effective treatment options for PVS after pediatric LT, most authors agree that stent insertion should be preferred in cases with potential need for early reintervention.[22, 23, 32]
For intraoperative and immediate postoperative PV complications, hybrid strategies combining a surgical approach with catheter-based angioplasty have been successfully used as well.[16-18] However, considering potential hemodynamic instability as well as worse imaging quality compared with a pediatric catheterization laboratory, these strategies should be reserved for bailout situations.
No published data exist on risk factors for recurrent stenosis after portal angioplasty for posttransplantation PVS treatment. Considering that low body weight and age at LT have been identified as risk factors of PV complications (Table 3),[2, 4, 21] we hypothesized that the same factors would result in a higher rate of restenosis rate after intervention. Surprisingly, neither one of those factors nor symptoms of fixed portal hypertension could predict if or when a patient would need reintervention sooner than others (Figure 3).
Stent migration of self-expanding stents have been described in the literature as potential long-term complications after angioplasty of PVs.[33] However, to the best of our knowledge, there is no report of stent migration after stent angioplasty with balloon expandible stents to treat posttransplantation PVS. In 15 years of interventional stenting of the PV, we did not observe any case of stent migration during f/u imaging.
Postinterventional medication may also influence primary patency of PV stents. Our early patients 15 years ago received acetylsalicylic acid monotherapy like other PTA patients. Over the years, the benefits of dual antiplatelet therapy in adult cardiologic interventions were adapted for pediatric angioplasty. In our center, we used a combination of clopidogrel and acetylsalicylic acid for patients considered at risk for postinterventional in-stent stenosis or thrombosis. Although there are no published data for primary stent placement, Sanada et al. even suggested a strategy of triple anticoagulant therapy with low-molecular-weight heparin, warfarin, and acetylsalicylic acid[34] in their study group of pediatric patients with PTBA (age 1.1–19.8 years). In their study, no recurrent PVS was seen over a f/u of 9 months to 3.9 years with triple therapy. However, larger balloons were used for angioplasty in this group, suggesting a bigger vessel size and, therefore, a better general prognosis.
Other authors identified jump graft or conduit reconstruction of the PV as risk factors for posttransplantation PVS.[5, 35] However, the type of anastomosis is usually determined by the available vasculature, such as short pedicles or extensive collateral vessels. In our cohort, reintervention was necessary in more (7/13) patients with atypical PV anastomoses. However, the small number of affected patients prevented further statistical analysis.
Our study has some limitations. First, this study was retrospective, so risk factors for PV complications could not be adequately analyzed and identified. Second, the data set is incomplete in some patients. Third, the low number of patients receiving PTBA did not allow for statistical comparison of PTBA and PTSA.
CONCLUSION
Taken together, we report 46 percutaneous interventions in 44 patients with posttransplantation PVS, of which 40 could be successfully treated with percutaneous primary stent angioplasty. The technical success rate of the intervention was 100%. Only five patients developed recurrent PVS during f/u, which again could be managed interventionally.
Our data demonstrate that primary PTSA is a safe and effective treatment for PVS after pediatric LT. All stents remained patent until the end of the f/u period, and primary patency was independent of age or weight at both transplantation and intervention.
Primary stent placement may constitute a long-term successful treatment option in most patients, including infants. As patients with LT have an almost average life expectancy, it remains an open question whether PTSA, especially at a young age, can provide lifelong PV patency. Reinterventions will undoubtedly be necessary for a subset of patients over time. However, angioplasty techniques using high-pressure balloons and stent-in-stent techniques offer treatment of recurrent PVS and may prevent the need for surgical reintervention.
ACKNOWLEDGMENT
Open access funding enabled and organized by Projekt DEAL. [Correction added on 16th June 2023, after first online publication: Mila Bukova was designated as corresponding author and Projekt DEAL funding statement has been added.]
CONFLICT OF INTEREST
Christoph M. Happel consults for Occlutech. Eva-Doreen Pfister advises for Orphalan, Albireo, Alexion, and Univar.