Triple-Phase Computed Tomography May Replace Dual-Energy X-ray Absorptiometry Scan for Evaluation of Osteoporosis in Liver Transplant Candidates
Abstract
Assessment of bone density is an important part of liver transplantation (LT) evaluation for early identification and treatment of osteoporosis. Dual-energy X-ray absorptiometry (DXA) is currently the standard clinical test for osteoporosis; however, it may contribute to the appointment burden on LT candidates during the cumbersome evaluation process, and there are limitations affecting its accuracy. In this study, we evaluate the utility of biomechanical analysis of vertebral images obtained during dual-energy abdominal triple-phase computed tomography (TPCT) in diagnosing osteoporosis among LT candidates. We retrospectively reviewed cases evaluated for LT between January 2017 and March 2018. All patients who underwent TPCT within 3 months of DXA were included. The biomechanical computed tomography (BCT) analysis was performed at a centralized laboratory (O.N. Diagnostics, Berkeley, CA) by 2 trained analysts blinded to the DXA data. DXA-based osteoporosis was defined as a T score ≤−2.5 at the hip or spine. BCT-based osteoporosis was defined as vertebral strength ≤4500 N for women or ≤6500 N for men or trabecular volumetric bone mineral density ≤80 mg/cm3. Comparative data were available for 91 patients who had complete data for both DXA and BCT: 31 women and 60 men, age 54 ± 11 years (mean ± standard deviation), mean body mass index 28 ± 6 kg/m2. Using DXA as the clinical reference, sensitivity of BCT to detect DXA-defined osteoporosis was 83.3% (20/24 patients) and negative predictive value was 91.7%; specificity and positive predictive value were 65.7% and 46.5%, respectively. BCT analysis of vertebral images on triple-phase computed tomography, routinely obtained during transplant evaluation, can reliably rule out osteoporosis in LT candidates. Patients with suspicion of osteoporosis on TPCT may need further evaluation by DXA.
Abbreviations
-
- BCT
-
- biomechanical computed tomography
-
- BMD
-
- bone mineral density
-
- BMI
-
- body mass index
-
- CT
-
- computed tomography
-
- DXA
-
- dual-energy X-ray absorptiometry
-
- FBS
-
- fragile bone strength
-
- HCC
-
- hepatocellular carcinoma
-
- LT
-
- liver transplantation
-
- NPV
-
- negative predictive value
-
- PPV
-
- positive predictive value
-
- TPCT
-
- triple-phase computed tomography
-
- TVBMD
-
- trabecular volumetric bone mineral density
Osteoporosis is a common complication of chronic liver disease, particularly in patients with cirrhosis and cholestatic liver diseases.(1) The pathophysiology of osteoporosis in the setting of chronic liver disease is multifactorial, including but not limited to low serum levels of insulin-like growth factor 1,(2) vitamin D deficiency,(3) negative impact of bilirubin on osteoblast function,(4) and uncoupling of bone resorption and bone formation.(5) Therefore, among patients with cirrhotic-stage liver disease, the prevalence of osteoporosis can exceed 50%.(5) In contrast to other complications of liver disease, osteoporosis is not cured with transplantation, and prior studies have identified acceleration of bone loss within the first few months of liver transplantation (LT), possibly owing to corticosteroid use during this timeframe.(6, 7) As a result, osteoporotic fractures can occur early after transplantation.(8)
The American Association for the Study of Liver Diseases and the American Society of Transplantation recommend testing for and treatment of osteoporosis prior to transplantation to prevent posttransplant decline of bone mineral density (BMD) and fractures.(9) Dual-energy X-ray absorptiometry (DXA) is currently the standard test used to diagnose osteoporosis.(10) Despite its widespread use, there are several limitations to DXA, including only modest sensitivity in predicting fracture risk.(11-14) In addition, DXA scanning lacks the ability to distinguish cortical from trabecular bone and can be further confounded by the presence of aortic calcification and certain bone abnormalities, including arthritic changes and osteophytes.(15) These limitations have led to a growing interest in computed tomography (CT)–based assessment of osteoporosis which studies thus far indicate that it can be a comprehensive and reliable means of assessing osteoporosis and fracture risk.(16) More particular to the context of LT, DXA scanning is part of the appointment burden during the overwhelming transplant evaluation. To date there have been no studies evaluating the possibility of using triple-phase CT (TPCT), routinely performed prior to transplantation, to assess osteoporosis. In this study, we evaluate the utility of TPCT in diagnosing osteoporosis among patients with cirrhosis undergoing evaluation for LT.
Patients and Methods
Study Design and Population
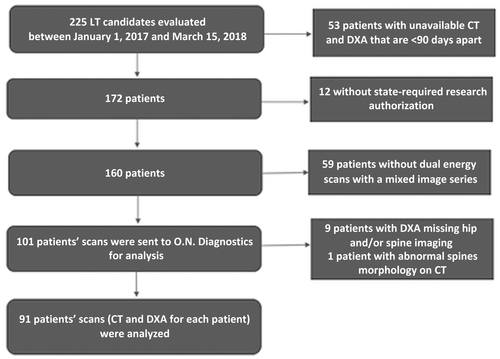
This is a retrospective review of charts of adult patients who underwent evaluation for LT at the Mayo Clinic in Rochester, Minnesota, between January 1, 2017, and March 15, 2018. The standard transplant evaluation at this center includes a dual-energy, contrast-enhanced TPCT to assess vascular anatomy and screen for hepatocellular carcinoma (HCC) and DXA to evaluate for osteoporosis. Our study included patients who underwent TPCT within 3 months of DXA. Patients were excluded if their TPCT and DXA were more than 90 days apart, their DXA exams were missing either hip or spine measurements, or if they had abnormal spine morphology. The patients’ charts were also excluded from the review if there was no state-required research authorization on file. The patient selection process is depicted in Fig. 1. Osteoporosis was defined as BMD T score ≤2.5 on DXA at either the hip or spine. The study was approved by the institutional review board.
Data Collection
The list of patients evaluated for LT during the observation period was obtained from our prospectively collected transplant center database. Clinical data such as age, sex, and body mass index (BMI) were collected by manual chart review. DXA reports were individually reviewed, and the following information was manually collected: (1) femoral neck BMD, femoral neck T score, total hip T score, and total hip BMD on each side and (2) vertebral BMD at L1, L2, L3, and L4 individually as well as total spine BMD and total spine T score. Deidentified, coded TPCT images were exported to the analysis team at O.N. Diagnostics after establishing an interinstitution data transfer agreement.
DXA
DXA was performed on General Electric (GE) Healthcare Lunar iDXA scanners for both the hip and spine.
TPCT
CT exams were acquired on dual-energy Siemens SOMATOM Definition Flash (Siemens Medical Solutions USA, Inc, Malvern, PA, USA) and SOMATOM Force scanners (Siemens Medical Solutions USA, Inc, Malvern, PA, USA) using a contrast-enhanced triple-phase protocol (late arterial to visualize tumor arterial enhancement, venous to look for complications of portal hypertension [such as portosystemic shunts and gastric and esophageal varices], and delayed to visualize tumor washout), with dual-energy acquisitions acquired during the late arterial and delayed phases of enhancement. For these exams, intravenous iodinated contrast is administered using a weight-based table, with a similar patient size-based table used for selecting the X-ray tube energies. Prior work in the enteric phase of enhancement has shown that BCT-based osteoporosis estimation is highly accurate in contrast-enhanced CT.(17) We selected the delayed phase for BCT analysis because we felt marrow enhancement would be minimized compared with the late arterial and portal phases. Mixed kV images were chosen for BCT analysis as opposed to other dual-energy reconstructions because of their CT number accuracy and lack of need for additional postprocessing. Images were reconstructed at 3-mm slice thickness using the Q30 (Flash) and Br44 (Force) kernels and a linear blend ratio of 0.6 (ie, 60% of the image coming from the low-energy X-ray tube and 40% from the high-energy X-ray tube). Reconstruction field of view was adjusted according to patient size (380 mm on average).
BCT Analysis
Finite element analyses and trabecular volumetric density (mg/cm3) measurements were performed on the CT scans by 2 trained analysts at O.N. Diagnostics blinded to the DXA data using VirtuOst software (O.N. Diagnostics, Berkeley, CA). The repeatability precision between 2 analysts analyzing the same scan (interoperator) for spine strength and trabecular density is 0.5 coefficient of variation % as illustrated in the prior study by Lee et al.(18) In that study, 25 women and 15 men (mean age ± standard deviation, 67 ± 9 years; range, 41-86 years) were analyzed, 1 scan per anatomic site per patient, and the scans were analyzed independently by 2 analysts using the VirtuOst software (O.N. Diagnostics)—as in the current study. The scans were acquired at 120 kVp, with a slice thickness/increment of 3 mm or less, on 9 different CT scanner models across 24 different scanners. The precision for the same analyst analyzing the same scan (intraoperator) was not measured in that study, but is expected to be at least as good as the interoperator precision. With regard to additional contribution of O.N. Diagnostics affiliates to the study, T.M.K. assisted with the clinical interpretation, which was led by the clinicians on the study team, and D.C.L. assisted with the technical interpretation of BCT.
Briefly, the L1 vertebra was segmented, and voxel intensity values were converted to BMD using a phantomless calibration (Fig. 2). The bone volume was then resampled into isotropic voxels (1 × 1 × 1 mm), and each voxel was then converted into a hexahedral finite element and assigned material properties based on empirical relationships with BMD. Displacement boundary conditions simulated uniform axial compression applied through a virtual layer of bone cement. Vertebral strength (N) was defined as the compressive force at 2% deformation. Trabecular volumetric bone mineral density (TVBMD) was determined using the same software, which was defined as the average density of an ellipsoidal volume placed inside the trabecular compartment in the central 10-mm section of the vertebral body.

Statistical Analysis
After the BCT testing was performed on all scans received, the BCT results were sent to the Mayo Clinic for data lock, and the DXA results were released for statistical comparisons with the BCT results. DXA-based osteoporosis was defined in the following 2 ways: T score ≤−2.5 at either the hip or the spine and at the hip only. CT-based osteoporosis was defined in the following 3 ways: using only TVBMD measurement (≤80 mg/cm3), using only strength measurement (≤4,500 N for men, ≤6,500 N for men), and using either measurement (either TVBMD ≤ 80 mg/cm3 or strength ≤4,500 N for women, ≤6,500 N for men). The 2 types of DXA-based osteoporosis classifications were compared against the 3 types of BCT-based classifications. The reclassification analysis using DXA as a standard and values of agreement, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), kappa statistic, and prevalence of osteoporosis were calculated. A t test was used to compare difference between means in patients with and without osteoporosis categories (nonparametric tests were used to confirm t tests with small sample sizes). All statistical analyses were performed using JMP (version 9, SAS, Cary, NC).
Results
Among all patients who underwent LT evaluation during the study period, 91 patients met the inclusion and exclusion criteria (Table 1); 31 of those included were women. Mean age for women and men was 55.8 and 52.9 years of age, respectively. Mean BMI of participants was comparable for women and men (26.5 versus 28.2 kg/m2) (Table 1).
| With DXA-Defined Osteoporosis (n = 24) | Without DXA-Defined Osteoporosis (n = 67) | Pooled (n = 91) | |
|---|---|---|---|
| Sex | Female = 9 | Female = 22 | Female = 31 |
| Male = 15 | Male = 45 | Male = 60 | |
| Age, years | 59.1 ± 13.1 | 54.5 ± 11.9 | 55.8 ± 12.3 |
| 54.3 ± 8.6 | 52.4 ± 11.8 | 52.9 ± 11.0 | |
| BMI, kg/m2 | 23.6 ± 5.2 | 27.7 ± 7.8 | 26.5 ± 7.3 |
| 25.8 ± 4.0 | 29.0 ± 5.2* | 28.2 ± 5.1 | |
| Time between DXA and CT, days | 4.3 ± 6.4 | 8.0 ± 17.2 | 6.9 ± 14.9 |
| 7.3 ± 15.9 | 13.1 ± 23.6 | 11.6 ± 21.9 | |
| DXA hip T score | −2.6 ± 0.4 | −1.2 ± 1.1† | −1.6 ± 1.1 |
| −2.5 ± 0.6 | −0.6 ± 0.9† | −1.1 ± 1.1 | |
| DXA spine T score | −2.8 ± 1.1 | −0.8 ± 1.5† | −1.4 ± 1.6 |
| −2.7 ± 0.8 | −0.1 ± 1.4† | −0.8 ± 1.7 | |
| BCT spine strength, N | 4350 ± 1240 | 5440 ± 1960 | 5130 ± 1830 |
| 4980 ± 1110 | 7650 ± 2520† | 6980 ± 2520 | |
| BCT trabecular volumetric bone mineral density, mg/cm3 | 95 ± 34 | 111 ± 35 | 106 ± 35 |
| 79 ± 30 | 115 ± 32† | 106 ± 35 |
- * P < 0.002.
- † P < 0.001.
Osteoporosis by DXA was present in 24 of 91 patients with a prevalence of 26.4%. The prevalence was 18.7% when CT vertebral images were analyzed with TVBMD measurements with the threshold value of 80 mg/cm3 and 47.3% with fragile bone strength (FBS) assessment with the threshold value of 100% (percentage of the fragile bone strength value [4500 N for women, 6500 N for men]). Figure 3 depicts the performance of these 2 analyses compared with DXA in a scatter plot format for male and female patients. In the case of DXA, the prevalence of osteoporosis was 29% in women and 25% in men, whereas for BCT it was 48.4% in women and 46.7% for men.
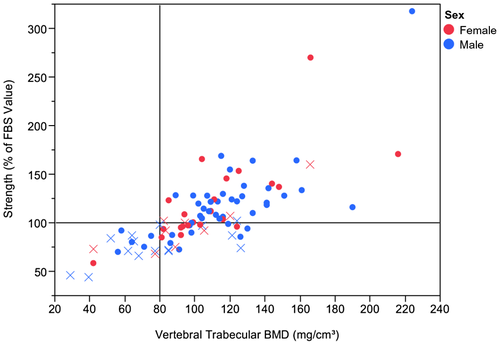
Separate analyses were performed for spine osteoporosis by TVBMD and by FBS and either TVBMD or FBS using the same 91 patients with both hip and spine DXA and spine BCT. There was 79.1% agreement of DXA with TVBMD and 70.3% agreement with FBS, with kappa scores 0.4 (±0.1) and 0.4 (±0.09), respectively.
Sensitivity of analyzed vertebral CT images was better with FBS at 83.3% (20/24 patients) compared with TVBMD at 45.5%. Of those 4 patients, 3 had borderline negative testing by BCT. The specificity was better with TVBMD at 91.0% compared with FBS at 65.7%. NPV to exclude osteoporosis with either TVBMD or FBS was 91.7% (Table 2).
| Patients With DXA BMD T Score ≤−2.5 (at Either Hip or Spine) | TVBMD | FBS | TVBMD or FBS |
|---|---|---|---|
| Accuracy, %* | 79.1 | 70.3 | 70.3 |
| Sensitivity, % | 45.8 | 83.3 | 83.3 |
| Specificity, % | 91.0 | 65.7 | 65.7 |
| PPV, % | 64.7 | 46.5 | 46.5 |
| NPV, % | 82.4 | 91.7 | 91.7 |
| Kappa score ± standard error | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| BCT prevalence, % | 18.7 | 47.3 | 47.3 |
| DXA prevalence, % | 26.4 |
| Patients With DXA BMD T Score ≤−2.5 (at the Hip Only) | TVBMD | FBS | TVBMD or FBS |
|---|---|---|---|
| Accuracy, %* | 80.2 | 64.8 | 64.8 |
| Sensitivity, % | 46.7 | 86.7 | 86.7 |
| Specificity, % | 86.8 | 60.5 | 60.5 |
| PPV, % | 41.2 | 30.2 | 30.2 |
| NPV, % | 89.2 | 95.8 | 95.8 |
| Kappa score ± standard error | 0.3 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| BCT prevalence, % | 18.7 | 47.3 | 47.3 |
| DXA prevalence, % | 16.5 |
NOTE:
- For DXA, osteoporosis was defined as BMD T score ≤−2.5 at the hip or spine. For BCT, only the spine was analyzed, and separate analyses were performed for defining osteoporosis by TVBMD, FBS, and either.
- * Accuracy = (number of true positives + number of true negatives)/total number of patients.
Discussion
The present study reveals novel observations that expand our diagnostic toolbox for osteoporosis in patients with cirrhosis. In particular, this study demonstrates the utility of using postprocessing of CT images obtained during abdominal triple phase abdominal CT for HCC screening in evaluating osteoporosis. This additional analysis of vertebral images (also known as BCT) on TPCT evaluated osteoporosis in LT candidates with a NPV exceeding 90% and a sensitivity exceeding 80%.
These findings suggest that BCT can eliminate the need for pretransplant DXA in a large proportion of LT candidates in whom osteoporosis was ruled out by this technique. In patients whose BCT suggests osteoporosis, further evaluation using DXA would be needed to further investigate these findings and to establish baseline DXA measurements, which would be used to assess response to osteoporosis therapy. A major advantage of this stepwise approach is the convenience to the patient and reduction in testing burden because TPCT is already a part of standard-of-care testing for LT candidates; therefore, incorporating these CT assessments requires no additional burden during the cumbersome transplant evaluation process. Although DXA per se is a simple and relatively quick test, the cumulative time assigned to the test during the evaluation process after accounting for registration, waiting, test performance, and checkout is about an hour, which is the time window reserved for DXA on the patients’ schedule in our institution. If the patient is a working individual, such additional appointment and/or visit, particularly when not combined with other tests on the same day, can have further financial implications such as losing a day or a half day of work. Therefore, if another test that is already routinely done (ie, TPCT) during the transplant evaluation can provide the information that is to be provided by DXA (ie, diagnosis of osteoporosis), it will be in the best interest of the patient’s convenience to eliminate DXA in this case. In addition, given that TPCT is frequently performed in nontransplant patients with chronic liver disease, measuring bone density at TPCT can increase the number of patients who are appropriately screened for osteoporosis, potentially preventing the morbidity associated with osteoporotic fractures.
In addition to patient convenience, this approach is safe because this special vertebral analysis of TPCT does not require additional radiation exposure or any alteration to the TPCT protocol. For patients whose BCT is suggestive of osteoporosis prompting referral to DXA for further evaluation, this combination of modalities (BCT and DXA) may be of benefit given the resultant comprehensive evaluation of bone density (by DXA and BCT) and bone strength (by BCT). Agten and colleagues demonstrated that a screening interval of every 5 years can suffice with combining BCT and DXA.(19) The combination was more cost-effective in relation to preventing future fractures compared with DXA alone or BCT alone. However, this simulation-based study was limited to postmenopausal women. These findings will need to be evaluated in LT recipients.
Prior studies evaluating BCT in comparison with DXA showed a high performance of BCT in predicting future fractures. Adam and colleagues showed that BCT assessment of hip and vertebral strength and density has higher sensitivity and specificity for predicting hip fracture compared with DXA—64% of the exams in this study were contrast enhanced.(20) Furthermore, Weber et al. showed in patients with inflammatory bowel disease that hip BCT based on contrast-enhanced CT enterography exams reliably identified patients with osteoporosis using DXA as the reference modality (sensitivity 85.7% and specificity 98.5%).(17) CT colonography, in another study, showed a NPV of 85.2% in ruling out osteoporosis compared with DXA.(21) The difference in the timing and phases of administering contrast between TPCT and CT assessments used in the aforementioned studies warranted the validation of BCT utility in TPCT used for HCC screening in patients with cirrhosis. In addition, this study is the first study to our knowledge to use contrast-enhanced, dual-energy images for BCT analysis. Dual-energy CT is used at many institutions for HCC screening because dual-energy images can increase the iodine signal at contrast-enhanced CT, thereby increasing the conspicuity of key imaging features of HCC such as arterial enhancement and tumor washout.(22-25) The current study was able to demonstrate this validation especially with regard to sensitivity and NPV and it suggests little impact on test performance when delayed-phase images are used.
With regard to post-LT monitoring of bone health, this approach can also be of benefit to a subset of patients. In 2015, more than 27% of LT recipients were transplanted for HCC.(26) In these patients, posttransplant surveillance for HCC is indicated, and most centers follow them with serial TPCT up to 5 years post LT. During a span of 5 years, some of these scans can be used for the evaluation of osteoporosis depending on the clinical scenario of the individual patients (eg, a patient with HCC who had negative BCT for osteoporosis pre-LT may have a repeat analysis on TPCT 3 years later).
The relatively limited agreement between BCT and DXA in our study merits further discussion of potential explanations. First is the heterogeneity across vertebrae as spine DXA measurement is a composite result for L1 to L4, whereas BCT analysis covers only 1 vertebral level (typically L1). As a result, there can be discrepancies if the lumbar vertebrae are not homogeneous in density or size. Second are the artifacts because DXA spine BMD can be influenced by artifacts such as aortic calcification and other degenerative features, deformities, or fractures, all of which are projected into the 2-dimensional DXA measurement. With the 3-dimensional CT-based measurement, only the bone of interest is segmented; therefore, aortic calcifications or bony features from adjacent vertebrae are excluded. Plus, we avoid making measurements in fractured/deformed vertebrae, which can have artificially high BMD. In this study, 7 patients had deformed/abnormal vertebra in L1; thus, we analyzed an alternate level. It is possible that some patients had a normal L1 (which we analyzed), but had deformities in L2, L3, and/or L4. We did not evaluate L2, L3, or L4 because in many cases the CT exam covers only L1 to L2. Third is that agreement is expectedly lower when osteoporosis is defined by both strength and density (as opposed to density only). This is because density by DXA and BCT does not take into account the bone's overall morphology and spatial distribution of bone density. By accounting for these 3-dimensional features, strength can better distinguish bones at risk of fracture—at the cost of disagreeing with the simpler density measurement. This point emphasizes the fact that DXA is not a gold standard but, rather, a clinical practice standard; therefore, disagreement with BCT in terms of properly classifying patients for osteoporosis and fracture risk may in fact reflect shortcomings of DXA rather than a problem with the BCT classification.
Despite the new insight provided by our results, there remain limitations to our study. It is a retrospective single-center study with a small sample size. The latter can potentially explain the difference in PPV between our study and prior studies. The relatively low PPV does not support the use of TPCT as a stand-alone osteoporosis test; therefore, we suggest the aforementioned stepwise approach where positive BCT-based testing is followed by DXA. We note that most of our population were men, which reflects the nature of our cohort being LT candidates,(27) whereas osteoporosis affects mostly women.(28) Another limitation is that because TPCT is an abdominal imaging modality that is not inclusive of the pelvis, we were not able to evaluate the femoral bone density and strength, although our study still showed remarkable NPV using vertebral BCT to predict osteoporosis of the hip or vertebra on DXA. This is not unexpected in view of prior data showing the evolution of osteoporotic changes starting at the vertebra before involving the hip and leading to hip fractures.(29) In fact, in a prior CT colonography-based study there was no patient with isolated hip osteoporosis (ie, without concurrent vertebral osteoporosis). Furthermore, L1 trabecular attenuation on CT has been shown in a prior study to be predictive of hip fracture.(30)
In summary, the routinely performed TPCT done for HCC screening in LT candidates is a reliable modality in ruling out osteoporosis in this patient population even when images are performed to maximize HCC detection using a dual-energy CT technique. However, patients with suspicion of osteoporosis on TPCT can be further evaluated with DXA. Larger studies are needed to validate these findings and to evaluate the predictability of BCT of future fracture risk and BCT utility during osteoporosis treatment in these patients.
Acknowledgment
The authors appreciate the assistance of Moen Taylor (Department of Radiology, Mayo Clinic, Rochester, MN) with processing the computed tomography images.