Chronic Kidney Disease in Liver Transplant Candidates: A Rising Burden Impacting Post–Liver Transplant Outcomes
Abstract
The burden of chronic kidney disease (CKD) is rising among patients with cirrhosis, though it is not known what impact this has had on outcomes after liver transplantation (LT). All patients listed for LT in the United States between 2002 and 2017 were analyzed, excluding those listed with Model for End-Stage Liver Disease (MELD) exceptions. The primary outcome was post-LT mortality. We defined pre-LT CKD as an estimated glomerular filtration rate <60 mL/minute for 90 days or ≥42 days of hemodialysis. Cox regression determined the association between pre-LT CKD and post-LT mortality. Of 78,640 LT candidates, the proportion with CKD among LT recipients increased from 7.8% in 2002 to 14.6% in 2017 (test for trend, P < 0.001). Among the 39,719 LT recipients, pre-LT CKD was significantly associated with post-LT mortality (hazard ratio [HR], 1.16; P < 0.001) even after adjusting for donor risk index (DRI), age, MELD, etiology, hepatic encephalopathy, simultaneous liver-kidney transplantation (SLKT), and diabetes. There was no mediating influence of SLKT on the effect of pre-LT CKD on post-LT survival (P > 0.05). Therefore, pre-LT CKD has a deleterious impact on post-LT outcomes, which is an impact that is not mediated through SLKT. These findings highlight the need for the identification of CKD when preventative measures are possible.
Abbreviations
-
- aHR
-
- adjusted hazard ratio
-
- AKI
-
- acute kidney injury
-
- ALD
-
- alcohol-related liver disease
-
- CKD
-
- chronic kidney disease
-
- CKD-EPI
-
- Chronic Kidney Disease Epidemiology Collaboration
-
- CI
-
- confidence interval
-
- DDLT
-
- deceased donor liver transplantation
-
- DRI
-
- donor risk index
-
- eGFR
-
- estimated glomerular filtration rate
-
- FHF
-
- fulminant hepatic failure
-
- HCV
-
- hepatitis C virus
-
- HD
-
- hemodialysis
-
- HE
-
- hepatic encephalopathy
-
- HR
-
- hazard ratio
-
- INR
-
- international normalized ratio
-
- IQR
-
- interquartile range
-
- LDLT
-
- living donor liver transplantation
-
- MELD
-
- Model for End-Stage Liver Disease
-
- MELD-Na
-
- Model for End-Stage Liver Disease–sodium
-
- NAFLD
-
- nonalcoholic fatty liver disease
-
- NASH
-
- nonalcoholic steatohepatitis
-
- OPTN
-
- Organ Procurement and Transplantation Network
-
- SLKT
-
- simultaneous liver-kidney transplantation
-
- UNOS
-
- United Network for Organ Sharing
Chronic kidney disease (CKD) is the end manifestation of persistent intrinsic renal damage, a pathology distinct from the reversible acute kidney injury (AKI) typically seen in patients with cirrhosis (eg, hepatorenal syndrome). This distinction is critical because a single measure of creatinine, as included within the Model for End-Stage Liver Disease (MELD) score, does not differentiate between AKI and CKD, and these entities significantly differ in how they impact short-term and longterm risk in patients with cirrhosis.1 Despite this important distinction, little is known of not only the burden of CKD among patients with cirrhosis but also of the impact of pre–liver transplantation (LT) CKD on post-LT outcomes.
In fact, studies evaluating the prevalence of CKD among patients with cirrhosis have largely focused on the increased utilization of simultaneous liver-kidney transplantation (SLKT).2-5 However, use of SLKT is highly variable, so this may not be the best metric to describe changes in the prevalence of CKD.2, 6, 7 This is critical because it is becoming more evident that pre-LT CKD impacts the risk of both pre-LT AKI and wait-list mortality.8, 9 That being said, although studies have investigated the impact of pre-LT renal function on post-LT survival, these studies were limited by subjective definitions of renal failure that did not distinguish between AKI and CKD.10-12 Consequently, it is not established if pre-LT renal function patterns differentially impact post-LT outcomes.
Herein, we aimed to find the prevalence of CKD among patients with cirrhosis on the LT waiting list and to determine the impact of CKD, as opposed to AKI, on post-LT mortality.
Patients and Methods
Patients
All patients listed for LT in the United Network for Organ Sharing (UNOS)/Organ Procurement and Transplantation Network (OPTN) registry between January 1, 2002, through December 31, 2017, were included in this study. Patients who were <18 years old, listed as status 1, who received exception points, or who underwent a living donor liver transplantation (LDLT) were excluded. We excluded those listed with exceptions because we believe that they are intrinsically different than those without exceptions with a different natural history of disease and therefore risk for CKD.
Renal Function
Serum creatinine and estimated glomerular filtration rate (eGFR) were determined longitudinally from the time of listing for LT to removal from the LT waiting list. Renal function was assessed every 7 days or longer. When a patient had more than 1 serum creatinine value for the same 7-day period, the first test result was used. We calculated eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine-based equation.13 We chose this equation because, of the glomerular filtration rate calculators that can be used with the data available in the UNOS/OPTN registry, the CKD-EPI equation most closely estimates glomerular filtration rate relative to glomerular filtration rate as measured by iothalamate clearance in patients with cirrhosis.14-17 Those on hemodialysis (HD) were treated as having an eGFR <15 mL/minute.
- AKI: A rise of serum creatinine ≥0.3 mg/dL or by >50% on the assessment prior to DDLT or having <42 days of HD at the time of DDLT.
- CKD: An eGFR <60 mL/minute for ≥90 days or having ≥42 days of HD at the time of DDLT.
- AKI on CKD: Meeting both criteria.
- Normal: Not meeting any of the above criteria.
Because of the nature of the UNOS database, we compared measurements for AKI within 7 days and not 48 hours as used in current definitions.20
- Stage 3: an eGFR at transplant <60 mL/minute but ≥30 mL/minute.
- Stage 4: an eGFR at transplant <30 mL/minute but ≥15 mL/minute.
- Stage 5: an eGFR at transplant <15 mL/minute or on HD.
Renal Function Pattern in Those Listed <90 Days
Because we could not know whether patients listed for <90 days had CKD, no patient listed for <90 days was considered to have CKD, unless they were on HD for ≥42 days. That being said, those patients listed for <90 days were eligible to meet the criteria for AKI.
Covariates
Data were obtained from the UNOS/OPTN registry as of April 6, 2018. Demographic data were collected at listing. The following data were collected at listing and at the end of follow-up: total bilirubin, international normalized ratio (INR), presence of hepatic encephalopathy (HE), and presence of ascites. Cutoffs deemed to be implausible were as follows: total bilirubin ≤0 mg/dL, INR ≤0, and creatinine ≤0 mg/dL.21 Observations with implausible values were set as missing for that specific value. The Model for End-Stage Liver Disease–sodium (MELD-Na) score22 was calculated and capped at 6 and 40, per current liver allocation policy. Because the MELD-Na score was not implemented until 2016, the MELD score was used in the descriptive statistics and analysis of the development of CKD.23, 24 Listing diagnoses were grouped into the following common diagnostic categories: hepatitis C virus (HCV), nonalcoholic fatty liver disease (NAFLD; including cryptogenic cirrhosis and nonalcoholic steatohepatitis [NASH]), alcohol-related liver disease (ALD), autoimmune etiologies (including primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis), and other. As has been done in previous studies,21, 25 regions were grouped according to median MELD score at time of LT into low (regions 3, 6, 10, and 11), medium (regions 2, 4, and 8), and high (regions 1, 5, 7, and 9) MELD regions. Donor characteristics included those used to calculate the donor risk index (DRI).26 The data were categorized into 4-year increments (2002-2005, 2006-2009, 2010-2013, and 2014-2017) based on the year of last follow-up to allow for an adequate sample size by year and to address changes in transplant policy (eg, implementation of MELD-Na and Share 35). The year of last follow-up was chosen to best determine the incidence of CKD at the time point closest to transplant.
Outcomes
The primary independent variable was the presence of CKD at the time of LT. A primary outcome of post-LT mortality was used to determine the impact of pre-LT CKD on post-LT survival. For this analysis, follow-up began on the date of LT and ended at the time of death or last update to the UNOS/OPTN registry.
Statistical Analysis
Descriptive Data Analysis
Continuous variables were compared between groups by Wilcoxon rank sum or Kruskal-Wallis tests. Categorical variables were compared between groups by chi-square test. To test for statistical trends over time, nonparametric tests for trend were used to evaluate significant changes in the percentage of patients by pre-LT year of last follow-up.
Cox Regression Analysis
Posttransplant patient survival was assessed using a Cox regression analysis. For this analysis, patient follow-up began on the date of LT and ended at the time of death, with those alive after transplant being censored at the time of last update of the UNOS registry (ie, March 6, 2019). To evaluate factors associated with post-LT mortality, Cox proportional hazards models were used. Unadjusted models were used to assess the association of covariates with the outcomes of interest. All covariates with a P < 0.2 in the univariate analysis were considered for inclusion in the multivariate models. Sequential backward selection was used to eliminate those not reaching a significance of P <0.05. Kaplan-Meier failure plots were generated to visualize the impact of the pre-LT final renal function pattern on post-LT survival. To highlight the importance of the distinction between AKI and CKD, we completed postestimation analyses of the final multivariate model to determine the average risk difference for post-LT mortality between the final pre-LT renal function patterns.
Mediation Analysis
We suspected that receiving an SLKT was a mediator in the effect of CKD on post-LT mortality. To explore this effect, we generated Cox regression multivariate models for overall post-LT survival. We determined the proportion of the adjusted effect of meeting the SLKT criteria on post-LT survival that was attributable to receiving an SLKT by comparing coefficient estimates of the 2 different models, with one not including the SLKT variable and the other including the SLKT variable. The 95% confidence intervals (CIs) for the proportion of this effect were calculated using the bias-corrected percentile bootstrap method with 1000 bootstrap samples.27 Meeting the SLKT criteria was defined as an eGFR <60 mL/minute for ≥90 days with an eGFR at LT of <30 mL/minute or as having ≥42 days of HD at DDLT.
Significance
Two-sided P values <0.05 were considered statistically significant. Analyses were performed using Stata, version 15.0 (StataCorp, College Station, TX). This study was approved by the institutional review board at the University of California, San Francisco.
Results
Population Characteristics
A total of 78,640 patients listed for LT met study inclusion criteria (Fig. 1). Of the 78,640 patients listed for LT, 39,719 (51%) received a DDLT, 20,791 (26%) either died on or were removed from the waiting list for “sickness,” and 18,130 (23%) were still waiting on the waiting list. Overall, the cohort consisted of 29,846 women (38%); 57,609 (73%) Caucasians; 18,589 (24%) patients with ALD; 27,289 (35%) patients with HCV; and 16,974 (22%) patients with NASH. Median age of the cohort at listing was 55 years (interquartile range [IQR], 48-60 years); median listing eGFR was 72 mL/minute (IQR, 43-98 mL/minute); median final eGFR was 57 mL/minute (IQR, 23-91 mL/minute); median listing MELD was 18 (IQR, 13-25); and median final MELD was 23 (16-32).
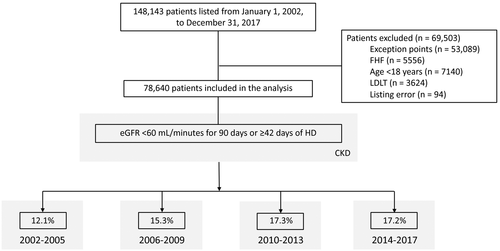
Among 39,719 DDLT recipients, 6269 (16%) patients met the CKD criteria at the time of last transplant (Table 1). Patients who developed CKD prior to LT were significantly older (58 versus 54 years; P < 0.001), more likely to be female (43% versus 34%; P < 0.001), and more likely to have a listing diagnosis of NAFLD/NASH (31% versus 21%; P < 0.001), ascites (55% versus 53%; P < 0.001), and diabetes mellitus (33% versus 20%; P < 0.001). They were also more likely to be from a high-MELD region (37% versus 32%; P < 0.001). Those who developed CKD had a significantly lower eGFR at listing (52 versus 72 mL/minute; P < 0.001; Table 1). During the study period, there were significant changes in the characteristics of patients undergoing LT. The proportion of patients meeting CKD criteria at the time of last follow-up increased from 7.8% in 2002 to 14.6% in 2017 (test for trend, P < 0.001; Fig. 2).
| No CKD (n = 33,450) | CKD (n = 6269) | P Value | |
|---|---|---|---|
| Age at last follow-up, years | 54 (47-60) | 58 (53-63) | <0.001 |
| Sex, female | 11,228 (34) | 2700 (43) | <0.001 |
| Ethnicity | <0.001 | ||
| Non-Hispanic white | 24,891 (74) | 4624 (74) | |
| African American | 3039 (9) | 492 (8) | |
| Hispanic | 4319 (13) | 829 (13) | |
| Asian | 790 (2) | 129 (2) | |
| Other | 411 (1) | 104 (2) | |
| Listing diagnosis | <0.001 | ||
| Alcohol | 7762 (23) | 1088 (17) | |
| HCV | 11,016 (33) | 2190 (35) | |
| NAFLD/NASH | 7139 (21) | 1970 (31) | |
| Autoimmune | 5207 (16) | 670 (11) | |
| Other | 2326 (7) | 351 (6) | |
| Region MELD | <0.001 | ||
| Low | 14,080 (42) | 2190 (35) | |
| Medium | 8815 (26) | 1771 (28) | |
| High | 10,555 (32) | 2308 (37) | |
| Ascites | 17,697 (53) | 3466 (55) | <0.001 |
| HE | 7920 (24) | 1410 (22) | <0.001 |
| Diabetes mellitus | 6319 (19) | 1928 (31) | <0.001 |
| MELD at listing | 21 (16-29) | 17 (14-21) | <0.001 |
| eGFR at listing | 72 (39-99) | 52 (35-72) | <0.001 |
| MELD at transplant | 25 (19-33) | 25 (20-32) | 0.001 |
| Received an SLKT | 2440 (7) | 1666 (27) | <0.001 |
| DRI per 0.1 point | 1.4 (1.2-1.8) | 1.4 (1.2-1.8) | 0.56 |
| Era of transplant | <0.001 | ||
| 2002-2005 | 6552 (20) | 903 (14) | |
| 2006-2009 | 8345 (25) | 1503 (24) | |
| 2010-2013 | 7802 (23) | 1632 (26) | |
| 2014-2017 | 10,751 (32) | 2231 (36) |
NOTE:
- Data are given as n (%) or median (IQR).
Cox Regression Analysis for Post-LT Survival
Of the 39,719 patients who underwent LT during the study period, 10,830 (27%) died after LT at a median of 2.3 years (IQR, 0.4-6.0 years). There was a significant decrease in both 1-year (12.4% in 2002 to 6.9% in 2016) and 5-year (23.9% in 2002 to 18.3% in 2012) post-LT mortality by year of transplant during the study period (P < 0.001 for both by test for trend). As compared with those without CKD, those with CKD prior to LT were significantly more likely to die after LT: 1-year mortality, 11.9% versus 9.0% (P < 0.001); and 5-year mortality, 21.5% versus 17.5% (P < 0.001).
In the univariate analysis for post-LT mortality, CKD at transplant was significantly associated with post-LT mortality (as compared with no CKD): stage 3 CKD, hazard ratio (HR), 1.16 (95% CI, 1.08-1.25); stage 4 CKD, HR, 1.42 (95% CI, 1.28-1.58); and stage 5 CKD, HR, 1.42 (95% CI, 1.31-1.54; Table 2).
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| CKD at transplant | 1.29 | 1.23-1.36 | <0.001 | 1.16 | 1.10-1.22 | <0.001 |
| Age per 1 year | 1.02 | 1.02-1.03 | <0.001 | 1.02 | 1.01-1.02 | <0.001 |
| Female sex | 0.97 | 0.93-1.02 | 0.22 | — | — | — |
| Etiology | ||||||
| Alcohol | — | — | — | — | — | — |
| HCV | 1.21 | 1.15-1.28 | <0.001 | 1.19 | 1.13-1.26 | <0.001 |
| NASH | 1.08 | 1.02-1.15 | 0.01 | 0.94 | 0.88-1.01 | 0.09 |
| Autoimmune | 0.79 | 0.74-0.85 | <0.001 | 0.82 | 0.76-0.88 | <0.001 |
| Other | 0.87 | 0.79-0.95 | 0.002 | 0.91 | 0.83-1.00 | 0.06 |
| Ethnicity | ||||||
| Non-Hispanic white | — | — | — | — | — | — |
| African American | 1.18 | 1.10-1.26 | <0.001 | 1.18 | 1.10-1.27 | <0.001 |
| Hispanic | 0.86 | 0.81-0.91 | <0.001 | 0.80 | 0.74-0.85 | <0.001 |
| Asian | 0.77 | 0.67-0.89 | 0.001 | 0.76 | 0.65-0.88 | <0.001 |
| Other | 1.03 | 0.86-1.22 | 0.78 | 1.01 | 0.84-1.22 | 0.91 |
| Ascites | 1.15 | 1.10-1.19 | <0.001 | — | — | — |
| HE | 1.24 | 1.19-1.29 | <0.001 | 1.16 | 1.11-1.22 | <0.001 |
| Diabetes mellitus | 1.35 | 1.29-1.42 | <0.001 | 1.33 | 1.26-1.40 | <0.001 |
| MELD at transplant per point | 1.01 | 1.01-1.02 | <0.001 | 1.02 | 1.01-1.02 | <0.001 |
| Received an SLKT | 1.23 | 1.16-1.31 | <0.001 | — | — | — |
| Era of transplant | ||||||
| 2002-2005 | — | — | — | — | — | — |
| 2006-2009 | 0.99 | 0.95-1.05 | 0.84 | 0.98 | 0.92-1.03 | 0.44 |
| 2010-2013 | 0.88 | 0.83-0.94 | <0.001 | 0.81 | 0.76-0.87 | <0.001 |
| 2014-2017 | 0.80 | 0.74-0.86 | <0.001 | 0.74 | 0.68-0.79 | <0.001 |
| DRI per 0.1 point | 1.03 | 1.02-1.03 | <0.001 | 1.03 | 1.03-1.04 | <0.001 |
- Age at transplant (HR, 1.02 per 1 year; 95% CI, 1.01-1.02).
- Etiology of cirrhosis compared with ALD for HCV (HR, 1.19; 95% CI, 1.13-1.26), NASH (HR, 0.94; 95% CI, 0.88-1.01), autoimmune-related (HR, 0.82; 95% CI, 0.76-0.88), and other (HR, 0.91; 95% CI, 0.83-1.00).
- Ethnicity compared with non-Hispanic white for African American (HR, 1.18; 95% CI, 1.11-1.27), Hispanic (HR, 0.80; 95% CI, 0.75-0.85), and Asian (HR, 0.76; 95% CI, 0.65-0.88).
- Presence of HE (HR, 1.16; 95% CI, 1.11-1.22).
- MELD at transplant (HR, 1.02 per 1 point; 95% CI, 1.01-1.02).
- Era of transplant compared with 2002-2005 for 2006-2009 (HR, 0.98; 95% CI, 0.92-1.03), 2010-2013 (HR, 0.82; 95% CI, 0.76-0.87), and 2014-2016 (HR, 0.73; 95% CI, 0.68-0.79).
- Presence of diabetes mellitus (HR, 1.32; 95% CI, 1.26-1.40).
- DRI (HR, 1.03 per 0.1 point; 95% CI, 1.03-1.04; Table 2).
Using this model, we discovered a number of additional findings. First, we found that any stage of CKD was associated with higher post-LT mortality, with the HR increasing at higher stages (as compared with no CKD): stage 3 CKD, HR, 1.11 (95% CI, 1.03-1.20); stage 4 CKD, HR, 1.19 (95% CI, 1.06-1.33); and stage 5 CKD, HR, 1.21 (95% CI, 1.11-1.32). Second, if instead of the diagnosis of CKD, the total days with an eGFR <60 mL/minute was incorporated into the final adjusted model, we found that for every 90 days with CKD, the risk of post-LT mortality increased by 2.7% (adjusted hazard ratio [aHR], 1.03; 95% CI, 1.02-1.04). Third, despite the significant improvement in LT outcomes by era, there was no significant interaction between the effect of pre-LT CKD on post-LT survival by era (P > 0.05 for all eras), meaning that we have not decreased the impact of pre-LT CKD on post-LT outcomes.
Mediating the Influence of Receiving an SLKT on post-LT Mortality in Those With CKD
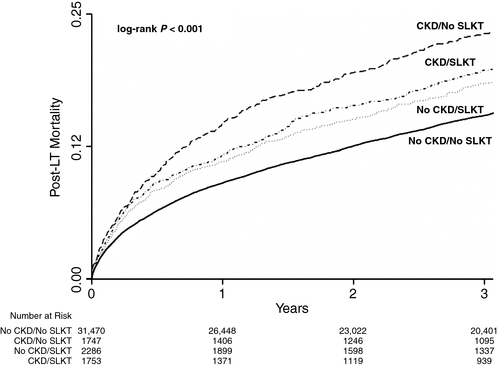
Next, we wanted to better understand the impact of receiving an SLKT on post-LT outcomes, particularly in the 3736 (9%) patients with CKD who met the SLKT criteria. Of those with pre-LT CKD, 48.6% received an SLKT versus 6.4% of those without (P < 0.001). There were significant differences between those who did and did not receive an SLKT (Supporting Table 1). The Kaplan-Meier failure plot demonstrates that those with any chronic renal dysfunction (eg, met SLKT without SLKT, SLKT without meeting SLKT, or met SLKT and underwent SLKT) had significantly higher rates of post-LT mortality (P < 0.001 by log-rank test; Fig. 3). Additional multivariate models then demonstrated that there was no significant mediating influence of SLKT on the effect pre-LT CKD had on post-LT survival: 1-year proportion of the attributable effect of SLKT (4.0%; bias corrected 95% CI, −41.1% to 49.1%); 5-year proportion of the attributable effect of SLKT (6.0%, bias corrected −9.7% to 23.1%).
Impact of the Renal Function Pattern on Post-LT Survival
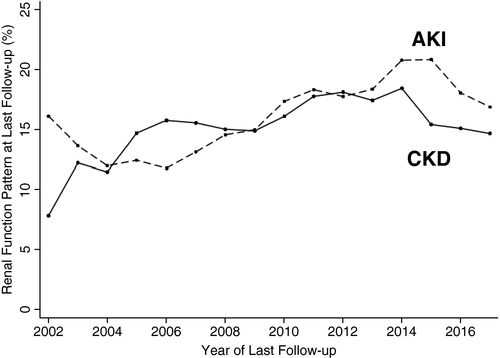
Of the 39,719 DDLT recipients, 5739 (14%) patients had AKI, 5188 (13%) patients had CKD, and 1019 (3%) patients had AKI on CKD at the time of their transplant. There was a significant trend for all renal dysfunction patterns (ie, AKI, CKD, and AKI on CKD) to increase during the study period (test for trend, P < 0.001). As compared with those patients with normal renal function, the rates of post-LT mortality were significantly higher in those with CKD (31% versus 26%; P < 0.001), AKI (29% versus 26%; P < 0.001), and AKI on CKD (31% versus 26%; P < 0.001).
In the univariate analysis, patients with CKD (HR, 1.33; 95% CI, 1.26-1.41), AKI (HR, 1.35; 95% CI, 1.27-1.42), and AKI on CKD (HR, 1.54; 95% CI, 1.37-1.73) had significantly higher risk of post-LT mortality as compared with patients with normal renal function at the time of LT. Similarly, in the final multivariate model after adjustment for age, MELD at transplant, etiology of cirrhosis, encephalopathy, diabetes at transplant, race, and era of transplant, each of the renal dysfunction patterns had greater post-LT mortality as compared with normal: AKI (aHR, 1.21; 95% CI, 1.13-1.27); CKD (aHR, 1.19; 95% CI, 1.12-1.27); and AKI on CKD (aHR, 1.28; 95% CI, 1.13-1.45).
That being said, there was no significant difference between the AKI or AKI on CKD groups and the CKD group (P > 0.05 for both comparisons).
Discussion
In this national study of more than 78,000 adult LT candidates in the United States, we aimed to describe and determine the impact of CKD among patients with cirrhosis undergoing LT. We first quantified the rise in the prevalence of CKD among LT recipients, demonstrating a 187% increase in the proportion of patients with cirrhosis who had CKD at the time of LT. Our data suggest that this rise has had a detrimental impact on post-LT outcomes, which is indicated by those patients with CKD at the time of transplant having an adjusted 16% increase in risk of death after LT. This increase is an effect that was independent of receiving an SLKT and equivalent to the impact of AKI on post-LT outcomes.
What might explain the rising prevalence of CKD? We offer 2 main reasons based on our results. First, we suspect that the emergence of NASH as a leading indication for LT has led to a greater proportion of patients with manifestations of metabolic syndrome being considered for transplant. As a result, these patients are more likely to have intrinsic renal damage (eg, diabetic nephropathy or hypertensive nephrosclerosis). They are therefore more susceptible to episodes of AKI and are less likely to have the renal reserve to recover from these episodes. This is supported by previous studies that demonstrated that those with a higher baseline creatinine are more susceptible to AKI and less likely to recover from those episodes of AKI.8, 9 Second, this increased susceptibility is potentiated by factors that lead to longer wait times, whereby patients who spend more time at risk for renal injury are most vulnerable to CKD. This not only leads to the progression of renal disease in those with intrinsic renal damage, but it also leaves the patient with decompensated cirrhosis exposed to hemodynamic abnormalities for a longer period, putting them at a greater risk for the development of type 2 hepatorenal syndrome.
The rising prevalence of CKD is all the more important given the association that we observed between CKD and mortality after LT. Although overall post-LT outcomes have improved over time,28 patients with CKD continued to have significantly higher rates of post-LT mortality in all eras, which is an impact that, on the basis of our data, was neither mitigated nor potentiated by SLKT. Moreover, we demonstrate that the deleterious impact of CKD on post-LT outcomes is directly correlated with the degree and duration of CKD, ie, the longer a patient has CKD in the pre-LT setting, the greater the impact of CKD on post-LT outcomes and the greater the CKD stage at transplant, the worse the post-LT survival. These findings suggest that the burden of CKD on the overall health of the LT recipient increases as renal function declines.
We acknowledge the following limitations to this study. First, we fully acknowledge that accurate ascertainment of all episodes of AKI would require serial measurements of creatinine at frequent and specific time points in all patients. However, our data reflect what is available in real-life clinical practice, and they demonstrate that AKI, as defined by what can be measured in this clinical setting, has prognostic value. Although the use of the UNOS/OPTN data set reflects the information that is currently available in clinical practice and thus enhances the generalizability of our findings to the real-life setting, our inability to determine those with CKD at the time of listing and the reliance on serum creatinine, which overestimates eGFR, means that we are likely underreporting the burden of CKD on the LT waiting list. Second, as with any analysis of UNOS registry data, our results are limited by the accuracy of the registry. We minimized any impact that clinical inaccuracy may have had in our results by focusing on objective data, such as serum creatinine. Third, because previous data suggested that patients listed with exception points (eg, hepatocellular carcinoma) have different causes of post-LT mortality, we excluded these patients from our analysis.29, 30 That being said, investigation is warranted into whether pre-LT CKD equally affects this group. Finally, there may be differences in patients with and without CKD, such as unmeasured comorbidities, that are not captured using national registry data. This highlights the need for prospective cohorts to better study renal disease among patients with cirrhosis.
Despite these limitations, our findings that CKD is rising among and having a greater burden on LT recipients has important implications for clinical practice. Specifically, these findings demonstrate the increasing importance of the appropriate management of CKD-associated medical comorbidities and the necessity to prevent the development and progression of CKD among LT candidates.