CAAT/enhancer binding protein–homologous protein deficiency attenuates liver ischemia/reperfusion injury in mice
Potential conflict of interest: Nothing to report.
Abstract
Ischemia/reperfusion injury (IRI) is one of the main causes of liver dysfunction after liver surgery. Involvement of endoplasmic reticulum (ER) stress in various diseases has been demonstrated, and CAAT/enhancer binding protein–homologous protein (CHOP) is a transcriptional regulator that is induced by ER stress. It is also a key regulator of ER stress-mediated apoptosis. The aim of this study was to investigate the role of CHOP in liver IRI. Wild type (WT) and CAAT/enhancer binding protein–homologous protein knockout (CHOP–/–) mice were subjected to 70% liver warm ischemia/reperfusion for 60 minutes. At different times after reperfusion, liver tissues and blood samples were collected for evaluation. Induction of ER stress including CHOP expression was ascertained. Liver damage was evaluated based on serum liver enzymes, liver histology, and neutrophil infiltration. Hepatocyte death including apoptosis was assessed. Liver warm IRI induced ER stress in both WT and CHOP–/– mice. In addition, CHOP expression was up-regulated in WT mice. At 6 hours after reperfusion, liver damage was attenuated in CHOP–/– mice. On the basis of terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling staining, apoptotic and necrotic cells were significantly reduced in CHOP–/– mice. CHOP deficiency also reduced the cleavage of caspase 3 and expression of the proapoptotic protein B cell lymphoma 2–associated X protein. Liver IRI induces CHOP expression, and CHOP deficiency attenuates liver IRI by inhibiting apoptosis. Elucidation of the function of CHOP in liver IRI may contribute to further investigation for a therapy against liver IRI associated with the ER stress pathway. Liver Transplantation 24 645–654 2018 AASLD.
Abbreviations
-
- ATF4
-
- activating transcription factor 4
-
- ALT
-
- alanine aminotransferase
-
- AST
-
- aspartate aminotransferase
-
- BAX
-
- B cell lymphoma 2–associated X protein
-
- BCL2
-
- B cell lymphoma 2
-
- BCL-xL
-
- B cell lymphoma extra large
-
- CHOP
-
- CAAT/enhancer binding protein–homologous protein
-
- CHOP–/–
-
- CAAT/enhancer binding protein–homologous protein knockout
-
- ER
-
- endoplasmic reticulum
-
- GRP78
-
- glucose-regulated protein 78
-
- H & E
-
- hematoxylin-eosin
-
- HPF
-
- high-power field
-
- HPRT
-
- hypoxanthine phosphoribosyltransferase
-
- IL
-
- interleukin
-
- I/R
-
- ischemia/reperfusion
-
- IRI
-
- ischemia/reperfusion injury
-
- MPO
-
- myeloperoxidase
-
- mRNA
-
- messenger RNA
-
- N.S.
-
- not significant
-
- RT-PCR
-
- real-time polymerase chain reaction
-
- SE
-
- standard error
-
- TUNEL
-
- terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling
-
- UPR
-
- unfolded protein response
-
- WT
-
- wild type
Liver ischemia/reperfusion injury (IRI) occurs in liver transplantation, liver resection (the Pringle maneuver or massive bleeding), and hemorrhagic and cardiogenic shock.1, 2 This results in liver dysfunction and failure, which can even lead to death via multiorgan system failure. Furthermore, tissue damage of the liver parenchyma induced by ischemia/reperfusion (I/R) can potentially lead to postoperative cancer recurrence.3-7
The forms of cell death that occur during liver I/R have been debated. On the basis of morphological assessment by hematoxylin-eosin (H & E) and terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling (TUNEL) staining, most hepatocyte death occurs via necrosis.8, 9 However, necrosis and apoptosis have common features and mechanisms. In addition, the initiation of the apoptotic process can be converted to necrosis upon adenosine triphosphate depletion at several stages of cell death. Moreover, it is sometimes difficult to distinguish between these 2 forms.10 On the other hand, Rüdiger et al. reported that apoptosis is the central mechanism of liver IRI.11 Additionally, many antiapoptotic strategies against liver IRI have been reported.12 Therefore, it is reasonable to approach liver IRI from the viewpoint of apoptosis.
- Up-regulation of ER chaperones to assist protein folding.
- Transcriptional attenuation.
- Degradation of unfolded proteins.
However, when ER stress surpasses this protective mechanism, apoptosis is induced. During ER stress-mediated apoptosis, transcriptional factor CAAT/enhancer binding protein–homologous protein (CHOP) plays an important role.17
In CAAT/enhancer binding protein–homologous protein knockout (CHOP–/–) mice, the improvement of various pathological conditions has been reported. Decreased liver damage in CHOP–/– mice has been shown in an intragastric ethanol feeding model,18 cholestasis-induced liver fibrosis model,19 and LPS-induced acute liver failure model.20 IRI in the myocardium,21 kidney,22-25 and neuronal cells26 was also shown to be attenuated in CHOP–/– mice. However, the relationship between CHOP and liver IRI has not been shown. Thus, the aim of this study was to clarify whether CHOP deficiency can attenuate liver IRI.
Materials and Methods
ANIMALS
Male C57BL/6 (age, 7-11 weeks; Japan SLC, Tokyo, Japan) and CHOP–/– mice (age, 7-11 weeks; C57BL/6 background) were used. CHOP–/– mice were provided by Professor Tomomi Goto (Kumamoto University, Kumamoto, Japan). Food and water were administered ad libitum, and mice were maintained under constant environmental conditions with a 12 hours/12 hours light-dark cycle. Protocols for all experiments were approved by the animal research committee of Kyoto University. Animals were cared for in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
LIVER WARM IRI MODEL
Mice were anesthetized by isoflurane inhalation and placed on a heating plate to maintain their body temperature during operation. After laparotomy, the vessels supplying 70% of the left side of the liver were disrupted using a micro atraumatic vascular clamp, and subsequently, the abdomen was closed. Before closure, sodium pentobarbital (60 mg/kg) was administered intraperitoneally for sedation during the period of ischemia. After 60 minutes, the clamp was removed, and reperfusion was initiated. Sham control mice underwent the same procedure without vascular occlusion. Mice were sacrificed at different time points after reperfusion, and liver tissue and blood were collected.
ASSESSMENT OF HEPATOCELLULAR DAMAGE
Serum aspartate aminotransferase (AST) and serum alanine aminotransferase (ALT) levels were measured to assess hepatocellular damage.
LIVER HISTOLOGY
Formalin-fixed, paraffin-embedded sections were cut at a thickness of 4 μm, and the slides were stained with H & E. Histological damage by liver I/R was blindly scored with Suzuki's criteria on a scale from 0 to 4.27
TUNEL ASSAY
TUNEL assays were performed on 4-μm sections using an Apoptosis in situ Detection Kit (Wako, Osaka, Japan) to detect apoptotic and necrotic cells in liver tissues 6 hours after reperfusion. The number of TUNEL-positive cells was counted in 10 randomly selected high-power fields (HPFs; magnification, ×400) per slide (n = 5 per group).
QUANTITATIVE REAL-TIME POLYMERASE CHAIN REACTION
Total RNA was extracted from liver tissue using TRIzol (Invitrogen Japan, Tokyo, Japan) and the RNeasy Mini Kit with on-column DNA digestion (Qiagen, Valencia, CA). In accordance with the manufacturers' protocol, total RNA was reverse transcribed to complementary DNA using an Omniscript RT Kit (Qiagen, Valencia, CA) with oligo (dT)8-12 primers (Invitrogen Japan). Quantitative real-time polymerase chain reaction (RT-PCR) was performed with a KAPA SYBR FAST qPCR Kit (Nippon Genetics, Tokyo, Japan) and StepOnePlus system (Applied Biosystems, Foster City, CA). The primers used in this study are shown in Table 1. Expression levels are provided as relative ratios to the expression level of hypoxanthine phosphoribosyltransferase (HPRT).
| Target | Forward | Reverse |
|---|---|---|
| CHOP | 5′-TATCTCATCCCCAGGAAACG | 5′-GGGCACTGACCACTCTGTTT |
| GRP78 | 5′-GAAAGGATGGTTAATGATGCTGAG | 5′-GTCTTCAATGTCCGCATCCTG |
| ATF4 | 5′-ATGGCCGGCTATGGATGAT | 5′-CGAAGTCAAACTCTTTCAGATCCATT |
| HPRT | 5′-TCAACGGGGGACATAAAAGT | 5′-TGCATTGTTTTACCAGTGTCAA |
WESTERN BLOT ANALYSIS
Western blot analysis was conducted as previously described,28 using the following primary antibodies and dilutions: anti-GADD153 (CHOP; number sc-7351; Santa Cruz Biotechnology, Santa Cruz, CA) at 1:200; anti-cleaved caspase 3 (number 9664; Cell Signaling Technology, Danvers, MA) at 1:1000; anti–B cell lymphoma 2–associated X protein (BAX; number 2772; Cell Signaling Technology) at 1:1000; anti–B cell lymphoma extra large (BCL-xL; number 2762; Cell Signaling Technology) at 1:1000; anti–B cell lymphoma 2 (BCL2; number sc-7382; Santa Cruz Biotechnology) at 1:500; and anti-β-actin antibody (number sc-47778, Santa Cruz Biotechnology) at 1:1000. The intensity of the bands was quantified with ImageJ.
IMMUNOHISTOCHEMISTRY
The specimens were fixed in 10% formalin and embedded in paraffin. Subsequently, they were deparaffinized, and endogenous peroxidase was quenched with 3% hydrogen peroxide in phosphate-buffered saline at room temperature for 10 minutes. The antigen was retrieved by incubation in a citric acid buffer at 120°C for 10 minutes. After being blocked, the sections were incubated with the primary antibody recognizing anti-CHOP (ab11419; Abcam) diluted at 1:100 at room temperature for 2 hours and anti-myeloperoxidase (MPO; ab9535; Abcam) diluted at 1:50 at 4°C overnight and then with labeled polymer in an envision + system horseradish peroxidase (Dako Japan, Tokyo, Japan) at room temperature for 1 hour. The sections were examined after incubation with a liquid 3,3′-diaminobenzidine substrate chromogen system (Dako Japan) for 5 minutes.
STATISTICAL ANALYSIS
All data are expressed as mean ± SE. Statistical analysis was performed using Student t test to compare mean values between groups. A P value < 0.05 was considered statistically significant. Analyses were performed by JMP pro 11 (SAS Institute, Cary, NC).
Results
CHOP EXPRESSION INCREASES IN WILD TYPE MICE FOLLOWING LIVER IRI
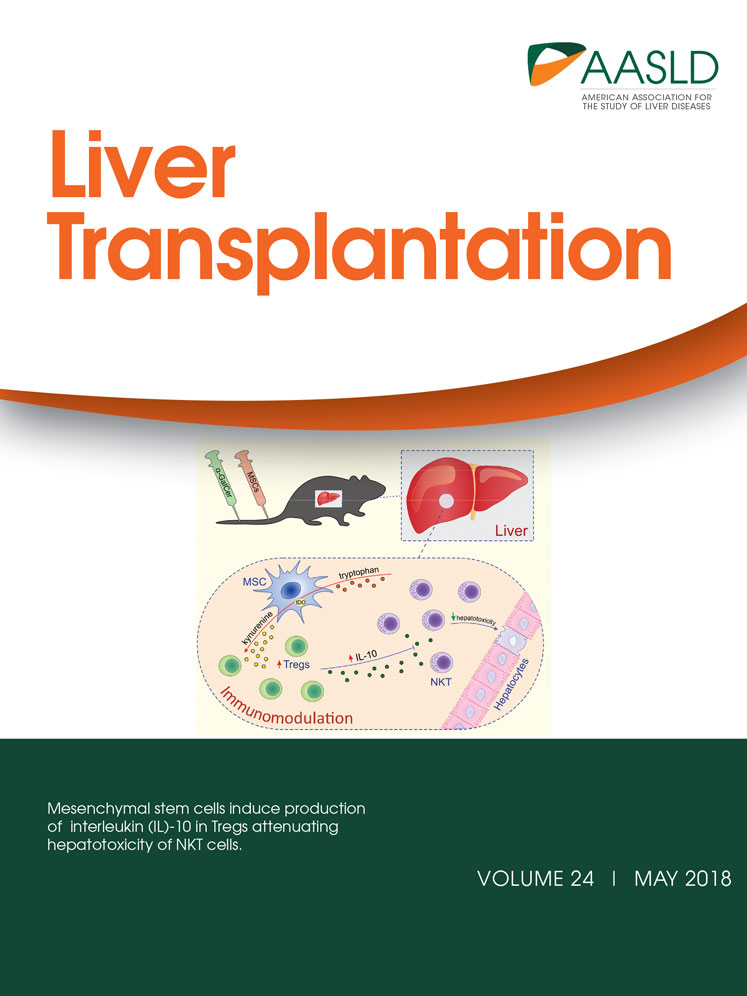
Wild type (WT) mice subjected to liver IRI were sacrificed 3 and 6 hours after reperfusion to ascertain whether this condition up-regulates CHOP expression in the mouse liver. RT-PCR demonstrated that CHOP messenger RNA (mRNA) expression increased by 16-fold at 3 hours after reperfusion compared with that in sham-operated mice (Fig. 1A). Consistent with mRNA levels, CHOP protein expression was elevated at 3 and 6 hours after reperfusion by Western blotting, whereas the CHOP protein was only minimally expressed in sham-operated mice (Fig. 1B). Then, to clarify which cell type within the liver up-regulates CHOP after I/R, we performed immunohistochemical staining for CHOP. In sham-operated livers, CHOP protein was faintly expressed in the cytosol of hepatocytes. Three hours after reperfusion, CHOP was strongly expressed in the cytosol and nucleus of hepatocytes, whereas nonparenchymal cells were not stained. At 6 hours after reperfusion, CHOP expression of hepatocytes decreased compared with that at 3 hours after reperfusion. CHOP expression of the cytoplasm diminished, whereas the nucleus stained strongly, and nonparenchymal cells also expressed CHOP (Fig. 1C).
IRI INDUCES ER STRESS IN BOTH WT AND CHOP–/– MICE
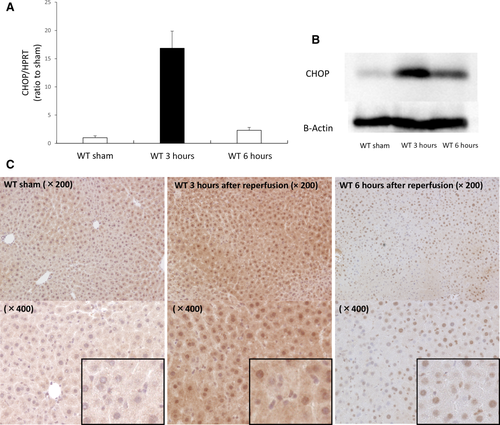
To confirm whether ER stress is induced by liver IRI in both WT and CHOP–/– mice, animals were sacrificed 3 and 6 hours after reperfusion. The ER stress marker glucose-regulated protein 78 (GRP78) was assessed by RT-PCR. GRP78 expression was significantly increased 3 hours after reperfusion in both WT and CHOP–/– mice. Three hours after reperfusion, GRP78 expression slightly increased in CHOP–/– mice, compared with that in WT mice (Fig. 2A). The activating transcription factor 4 (ATF4)-CHOP pathway, which is known to be the main pathway for CHOP induction, was also activated. The mRNA level of ATF4, which is upstream of CHOP, also increased 3 hours after reperfusion in both WT and CHOP–/– mice (Fig. 2B). Similar to CHOP expression, GRP78 and ATF4 mRNA expression peaked at 3 hours after reperfusion. These results indicated that liver IRI induces ER stress in both WT and CHOP–/– mice.
CHOP DEFICIENCY ATTENUATES LIVER IRI
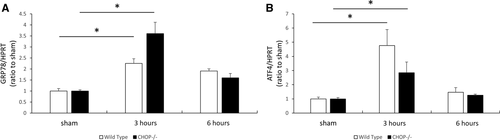
To test whether CHOP deficiency attenuates liver damage induced by I/R, liver sections and blood samples from animals 6 hours after I/R were evaluated. Serum AST and ALT levels were significantly increased in WT mice (ALT, 8438 ± 699 IU/L; AST, 3788 ± 313 IU/L) compared with that in CHOP–/– mice (ALT, 5084 ± 830 IU/L; AST, 2543 ± 260 IU/L) at 6 hours after reperfusion (P < 0.05; Fig. 3A). On the basis of H & E staining of livers 6 hours after reperfusion, extended necrotic areas and vacuolization were attenuated in CHOP-deficient mice compared with WT mice. The Suzuki score significantly decreased in CHOP–/– mice (Fig. 3B). Then, we assessed neutrophil infiltration induced by liver IRI by immunohistochemical staining for MPO. Numbers of MPO-positive cells were significantly decreased in the livers of CHOP–/– mice at 6 hours after reperfusion as compared with WT mice (Fig. 3C).
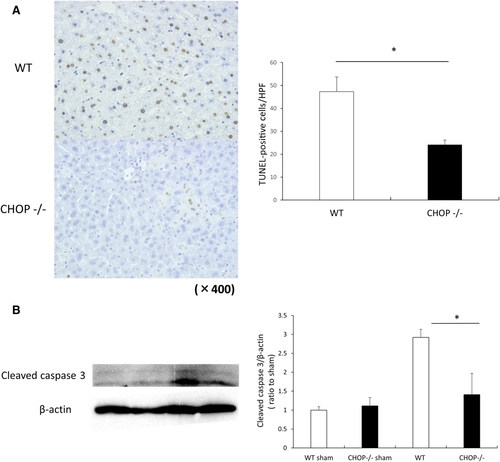
LIVER IRI–INDUCED APOPTOSIS AND NECROSIS ARE DECREASED IN CHOP–/– MICE
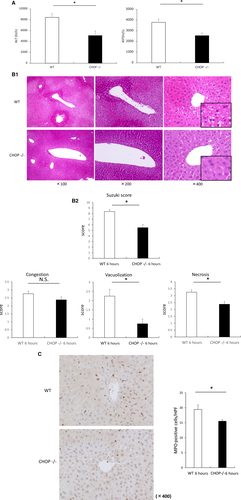
We next evaluated hepatocyte death induced by IRI at 6 hours after reperfusion in both WT and CHOP–/– mice. The number of TUNEL-positive cells was significantly decreased in CHOP–/– mice (47 ± 6 [WT] versus 24 ± 2 [CHOP–/–] per HPF; P < 0.05). Most of the TUNEL-positive cells were hepatocytes, and both the nucleus and cytoplasm were stained, demonstrating a staining pattern indicative of necrotic cells (Fig. 4A). To ascertain whether apoptosis was involved in this process, cleaved caspase 3 was analyzed by Western blotting. Cleaved caspase 3 was expressed at a low level in sham-operated mice. Cleaved caspase 3 expression increased after I/R in both groups of animals. However, the expression of this marker was significantly higher in WT mice than in CHOP–/– mice (Fig. 4B).
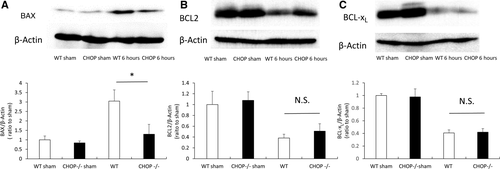
CHOP DEFICIENCY ATTENUATES HEPATOCYTE APOPTOSIS INDUCED BY IRI THROUGH THE INTRINSIC APOPTOSIS PATHWAY
The expression levels of BCL2 family proteins—BCL2, BCL-xL, and BAX—were determined by Western blotting. Compared with that in sham-operated mice, the expression of the antiapoptotic proteins BCL2 and BCL-xL decreased, and the expression of the proapoptotic protein BAX was increased at 6 hours after reperfusion. After IRI, BAX expression was significantly suppressed in CHOP–/– mice compared with that in WT mice (Fig. 5A). Meanwhile, BCL2 was expressed at a slightly higher level in CHOP–/– mice than in WT mice, and the expression of BCL-xL was virtually identical in both groups (Fig. 5B,C).
Discussion
Several relationships between ER stress and liver IRI have been reported. The small molecule chaperone 4-phenyl butyric acid, which ameliorates ER stress, improves liver IRI.29, 30 In addition, some drugs attenuate liver IRI via the ER stress pathway.31 Improvement of liver IRI by inhibiting the ER stress pathway, involving CHOP, has also been reported.32 Furthermore, in an animal model of traumatic hemorrhagic shock, which can be regarded as a mild liver I/R model, ER stress was induced to a greater extent than oxidative stress. This suggests a deep involvement of ER stress and liver IRI.33
The relationship between CHOP and IRI of other organs has also been reported. In the kidney and cerebellum, elevation of CHOP expression induced by IRI continues until after 24 hours,22, 34 whereas CHOP expression peaks at 0.5 hours after reperfusion in myocardial reperfusion injury.21 In our liver IRI model, CHOP expression peaked at 3 hours after reperfusion at mRNA and protein levels. However, immunohistochemistry revealed that liver nonparenchymal cells expressed CHOP later than hepatocytes, in which CHOP expression peaked at 3 hours after reperfusion. Although improvement of liver injury was mild, we confirm that CHOP deficiency attenuates IRI of the liver as shown in other organs.
CHOP regulates the intrinsic apoptosis pathway via BCL2 family proteins, and these proteins, including BCL2, BCL-xL, and BAX, are involved in liver IRI.35-37 Our results show that liver IRI was attenuated in CHOP-deficient mice through the inhibition of BAX expression and the attenuation of apoptosis. The proapoptotic molecule BAX, which stimulates cytochrome c release from mitochondria and increases caspase 3 activity, occurs downstream of CHOP and plays an important role in CHOP-mediated apoptosis. In addition, BAX can induce apoptosis independent of other antiapoptotic molecules.35 Ben-Ari et al. showed that liver IRI was attenuated in BAX–/– mice.35 Recently, the relationship between CHOP and inflammation has been reported. In IRI, inflammatory cytokines such as tumor necrosis factor α, interleukin (IL) 6, and IL1β were suppressed in myocardia of CHOP–/– mice,21 and IL1β was also suppressed in kidney tissue of CHOP–/– mice.22 In a study of steatohepatitis, the relationship between CHOP and the nuclear factor kappa B pathway was shown in human primary hepatocyte.38 We assessed the inflammatory response including these cytokines and pathways, but we could not find a significant difference between the groups. However, the significant difference in neutrophil infiltration in both groups suggested the involvement of CHOP in some inflammatory response. One of the reasons for this result is probably the mildness of improvement of liver damage in this study.
However, it should be carefully considered whether inhibiting the UPR itself leads to alleviation of liver IRI in clinical practice. Zhou et al. showed that the role of the UPR in the mouse liver I/R model differs between a 30-minute ischemia group and a 90-minute ischemia group. Specifically, this response was pathogenic in the 90-minute ischemia model but was protective in the 30-minute group.39 This report reminds us that the UPR is originally a protective mechanism. In the clinical setting, severe liver damage, as induced in the animal model of warm IRI, is rare because liver resection is carefully performed to mitigate postoperative liver failure, and hemorrhagic and cardiogenic shock are not a complete interruption of the blood flow. During liver resection, moderate IRI in small remnants of the liver causes liver failure, not severe IRI itself. Portal clamping6 or intraoperative blood loss40, 41 might affect tumor recurrence even if they are not fatal during the acute phase. For these reasons, liver warm IRI needs to be improved, even if it does not readily occur with severity. Meanwhile, because the degree of liver damage is different from that of the animal model, we must carefully address the relationship between the UPR and liver IRI in clinical situations. Elucidation of the function of CHOP in liver IRI should facilitate the investigation of this relationship.
After renal IRI in CHOP–/– mice, several mechanisms for ameliorating injury have been reported.22-25 Noh et al. showed that the BAX/BCL2 ratio is significantly decreased in the I/R kidney in CHOP–/– mice.22 In contrast, Chen et al. did not find any significant difference in the expression of BAX and BCL2 between CHOP–/– and WT mice.25 Noh et al. suggested that this difference was due to the degree of kidney injury. In the future, further elucidation is also expected regarding the mechanism through which liver IRI is alleviated in CHOP–/– mice.22
In conclusion, we have for the first time shown that CHOP deficiency attenuates liver IRI. These results may contribute to further investigation of a therapy against liver IRI associated with the ER stress pathway.