Heparin but not tissue plasminogen activator improves outcomes in donation after circulatory death liver transplantation in a porcine model
Research was supported by FIS Grant 11/01979 from the Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación, Spain. Marina Vendrell was supported by the “Premio Fin de Residencia ‘Emil Letang’” from the Hospital Clínic, Barcelona.
Potential conflict of interest: Nothing to report.
Abstract
Ischemic-type biliary lesions (ITBLs) arise most frequently after donation after circulatory death (DCD) liver transplantation and result in high morbidity and graft loss. Many DCD grafts are discarded out of fear for this complication. In theory, microvascular thrombi deposited during donor warm ischemia might be implicated in ITBL pathogenesis. Herein, we aim to evaluate the effects of the administration of either heparin or the fibrinolytic drug tissue plasminogen activator (TPA) as means to improve DCD liver graft quality and potentially avoid ITBL. Donor pigs were subjected to 1 hour of cardiac arrest (CA) and divided among 3 groups: no pre-arrest heparinization nor TPA during postmortem regional perfusion; no pre-arrest heparinization but TPA given during regional perfusion; and pre-arrest heparinization but no TPA during regional perfusion. In liver tissue sampled 1 hour after CA, fibrin deposition was not detected, even when heparin was not given prior to arrest. Although it was not useful to prevent microvascular clot formation, pre-arrest heparin did offer cytoprotective effects during CA and beyond, reflected in improved flows during regional perfusion and better biochemical, functional, and histological parameters during posttransplantation follow-up. In conclusion, this study demonstrates the lack of impact of TPA use in porcine DCD liver transplantation and adds to the controversy over whether the use of TPA in human DCD liver transplantation really offers any protective effect. On the other hand, when it is administered prior to CA, heparin does offer anti-inflammatory and other cytoprotective effects that help improve DCD liver graft quality. Liver Transplantation 24 665–676 2018 AASLD.
Abbreviations
-
- AP
-
- alkaline phosphatase
-
- AST
-
- aspartate aminotransferase
-
- CA
-
- cardiac arrest
-
- cDCD
-
- controlled/expected donation after circulatory death
-
- CK19
-
- cytokeratin 19
-
- CS
-
- cold storage
-
- CT
-
- clotting time
-
- DBD
-
- donation after brain death
-
- DCD
-
- donation after circulatory death
-
- GGT
-
- gamma glutamyltransferase
-
- HAF
-
- hepatic artery flow
-
- IL
-
- interleukin
-
- ITBL
-
- ischemic-type biliary lesion
-
- LDH
-
- lactate dehydrogenase
-
- LY30
-
- lysis index at 30 minutes
-
- LY60
-
- lysis index at 60 minutes
-
- MCF
-
- maximum clot firmness
-
- mRNA
-
- messenger RNA
-
- NRP
-
- normothermic regional perfusion
-
- PAI-1
-
- plasminogen activator inhibitor-1
-
- PVF
-
- portal vein flow
-
- QPT
-
- quick prothrombin time
-
- ROTEM
-
- rotational thromboelastometry
-
- RT-PCR
-
- reverse transcriptase polymerase chain reaction
-
- TNF
-
- tumor necrosis factor
-
- TPA
-
- tissue plasminogen activator
-
- uDCD
-
- uncontrolled/unexpected donation after circulatory death
Because primary graft failure can generally be avoided by appropriate donor and graft selection, ischemic-type biliary lesions (ITBLs) are currently the most dreaded complication of donation after circulatory death (DCD) liver transplantation. ITBLs are defined as diffuse nonanastomotic biliary strictures, with or without prestenotic dilatations, in the presence of a patent hepatic artery,1 and they occur in approximately 16% of recipients of DCD livers.2 The development of ITBLs is associated with significantly increased patient morbidity due to the need for multiple biliary procedures and repeat hospitalizations.2, 3 Up to 65% of patients with ITBLs require retransplantation or die.3 Furthermore, the fear over the development of ITBLs significantly limits the utilization of livers arising through the DCD process,4, 5 wasting organ recovery resources and limiting access to this potentially lifesaving procedure.
Ischemia/reperfusion injury has primarily been implicated in ITBL pathogenesis.6 An additional theory holds that microthrombi deposition in peribiliary arterioles during the low-flow/no-flow period following ventilator withdrawal or cardiorespiratory arrest in controlled/expected donation after circulatory death (cDCD) and uncontrolled/unexpected donation after circulatory death (uDCD) donors, respectively, may also play a role. On the basis of this theory, different authors have suggested that the viability of organs arising from DCD donors may be improved through the application of fibrinolytic therapy.7-10
Normothermic regional perfusion (NRP) is used to limit warm ischemia during the period following declaration of death and prior to cold perfusion in uDCD and more recently cDCD donors.11 Normothermic regional perfusion has been shown to replete cellular energy stores, reduce nucleotide degradation products, and induce endogenous antioxidants. It also allows for a comprehensive assessment of organ viability prior to recovery.12 Furthermore, NRP offers an ideal opportunity to treat and improve the quality of these marginal grafts prior to recovery.
The aim of the present study is to compare and contrast the effects on liver graft quality of fibrinolytic therapy administered during a period of postarrest NRP with that of pre-arrest heparinization followed by postarrest NRP (our current standard for cDCD donor maintenance)13 and postarrest NRP only (the standard in some countries where pre-arrest heparinization is prohibited by law) in a porcine model of DCD liver transplantation.
Materials and Methods
ANIMALS
Male weanling pigs were used as donors and recipients. Animals were cared for according to the guidelines of the University of Barcelona Committee on Ethics in Animal Experimentation and the Catalan Department of the Environment Commission on Animal Experimentation.
STUDY OVERVIEW
- Control group: 60 minutes cardiac arrest (CA) + 1 hour NRP + 4 hours cold storage (CS) + transplantation (n = 6).
- Tissue plasminogen activator (TPA) group: 60 minutes CA + 1 hour NRP with TPA + 4 hours CS + transplantation (n = 6).
- Heparin group: pre-arrest heparinization + 60 minutes CA + 1 hour NRP + 4 hours CS + transplantation (n = 6).
DONOR PROCEDURE AND NRP
Donor anesthesia was performed as described previously.14 The liver and hilar structures were dissected, and cholecystectomy was performed. Hepatic artery flow (HAF) and portal vein flow (PVF) were measured using ultrasonic flow probes connected to a flowmeter (HT107, Transonic Systems, Ithaca, NY).
Control Group
The descending aorta was cross-clamped at the aortic hiatus, and the heart was stopped with an intravenous injection of potassium chloride. The abdomen was closed, and a heating pad was placed to maintain a core temperature of 37°C. Sixty minutes after the start of CA (the length of time chosen to maximize biliary injury), the abdomen was reopened, and the abdominal aorta and inferior vena cava were cannulated with a 16-Fr Jostra arterial cannula and a multiperforated 21-Fr HLS venous cannula, respectively (Maquet Cardiopulmonary, Hirrlingen, Germany). Cannulae were connected to a cardiopulmonary bypass circuit (Maquet Cardiopulmonary, Hirrlingen, Germany) primed with 500 mL of 1/6 M sodium bicarbonate, 500 mL of 20% mannitol, 500 mL of Ringer's solution, and 500 mL of Voluven (Fresenius Kabi, Bad Homburg, Germany). Heparin (3 mg/kg) was also added to the priming solution. Normothermic regional perfusion was run for 60 minutes, with a goal pump flow of 1.7-1.8 L/minute and temperature 37°C. HAF and PVF were recorded, and the perfusate was sampled via an in-line 3-way stopcock throughout NRP. At the end of NRP, the liver was perfused with 1 L of cold IGL-1 preservation solution (Institut Georges Lopez, Lissieu, France) both portally and arterially. The liver was then removed, prepared, and placed in CS at 4°C.
TPA Group
TPA was added to the regional perfusion solution (1.5 mg/kg, administered as a 10-mg priming dose and the rest as a continuous infusion over the subsequent hour of NRP). The remainder of the procedure was the same as that described for the control group.
Heparin Group
Heparin (3 mg/kg intravenously) was administered approximately 5 minutes prior to aortic cross-clamp; the remainder of the procedure was the same as that described for the control group.
RECIPIENT PROCEDURE AND POSTOPERATIVE MANAGEMENT
Approximately 4 hours after the start of CS, the liver was transplanted into the recipient pig, as described previously.14, 15 Postoperatively, the recipient was cared for intensively for up to 5 days.14 Animals that survived until the fifth day were reopened and euthanized under anesthesia, whereas nonsurvivors were subjected to autopsy.
BLOOD AND SERUM ANALYSES
In blood samples collected serially during follow-up, alkaline phosphatase (AP), aspartate aminotransferase (AST), gamma glutamyltransferase (GGT), total bilirubin, and the quick prothrombin time (QPT) were determined using the Advia 1650 automatic analyzer (Bayer, Tarrytown, NY).
ROTATIONAL THROMBOELASTOMETRY
Rotational thromboelastometry (ROTEM) analyses were performed on blood samples taken from both the donor (prior to and at the end of CA and during NRP) and the recipient after graft reperfusion. Whole blood was activated extrinsically with recombinant tissue factor (EXTEM reagent) or intrinsically with ellagic acid (INTEM reagent), and the analysis was run for at least 60 minutes. In the HEPTEM assay, coagulation is triggered via the intrisic pathway in the same manner as in INTEM. However, heparinase is added to rapidly degrade heparin and allow for the assessment of hemostasis in heparinized subjects. Alpha angle measured the speed of clot strengthening. Maximum clot firmness (MCF) was the maximum amplitude of the clot, whereas maximum lysis was the maximum percentage of amplitude lost relative to MCF (normal <15%, >15% indicating hyperfibrinolysis). The lysis index at 30 minutes (LY30) and the lysis index at 60 minutes (LY60) were the ratios of the amplitudes at 30 and 60 minutes, respectively, relative to MCF (normal 94%-100%).
TISSUE ANALYSES
Liver parenchymal biopsies were taken at baseline in the donor (immediately after opening the abdominal wall), and parenchymal and distal bile duct samples were taken both 1 hour after reperfusion in the recipient and at the end of follow-up. Each biopsy was divided into 2 sections: 1 preserved in 10% formalin for paraffin inclusion and 1 diced finely and snap-frozen in liquid nitrogen for RNA extraction. Formalin-fixed samples were processed for routine histology. Sections were stained with hematoxylin-eosin and examined using standard light microscopy by 2 independent observers blinded to the identity of each sample. Parenchymal sections were evaluated for sinusoidal congestion, hepatocellular vacuolization, and necrosis according to the semiquantitative method described by Suzuki et al.,16 and peribiliary arteriolonecrosis and gland injury were evaluated according to the methods described by Hansen et al. and op den Dries et al.17, 18
To study fibrin deposition, paraffinated sections were dewaxed with xylene and stained for fibrin using the Martius Scarlet Blue Stain Kit (Molekula, Newcastle Upon Tyne, UK), according to the manufacturer's instructions. Also in paraffin-embedded tissue samples, endogenous peroxidase activity was inhibited with peroxidase-blocking solution (S2023, DakoCytomation, Glostrup, Denmark) for 10 minutes. Sections were washed with phosphate-buffered saline and incubated with anti-cytokeratin 19 (CK19) antibody (1/300, NCL-CK19 Mouse Monoclonal Antibody, Leica Biosystems GmbH, Nussloch, Germany) for 60 minutes. Slides were washed with phosphate-buffered saline and incubated with secondary antibody (K4011, Dako Agilent Pathology Solutions, Glostrup, Denmark) for 30 minutes. The peroxidase reaction was developed with a 3,3-diaminobenzidine tetrahydrochloride substrate kit (K4007, Dako Agilent Pathology Solutions). Finally, slides were washed with distilled water and counterstained with hematoxylin-eosin and examined by 2 independent observers blinded to the identity of each sample.
Real-time quantitative TaqMan reverse transcriptase polymerase chain reaction (RT-PCR) analyses of E-selectin, interleukin (IL) 1β, IL6, and tumor necrosis factor (TNF) gene expression were performed to detect endothelial cell activation and inflammation, as described previously.15 All samples were assayed in duplicate, recording the mean for each sample. Results in each individual animal were normalized with the value of the respective gene in that animal at baseline, which was set to 0.
DATA AND STATISTICAL ANALYSES
All quantitative values are expressed as the median and 25%-75% interquartile range. Differences between groups were compared using the Mann-Whitney U test for 2 samples and the Kruskal-Wallis 1-way analysis of variance for multiple samples. P < 0.05 was considered significant. Calculations were performed using SPSS statistics, version 20 (IBM, Armonk, NY).
Results
DONOR OUTCOMES
Graft Perfusion During NRP
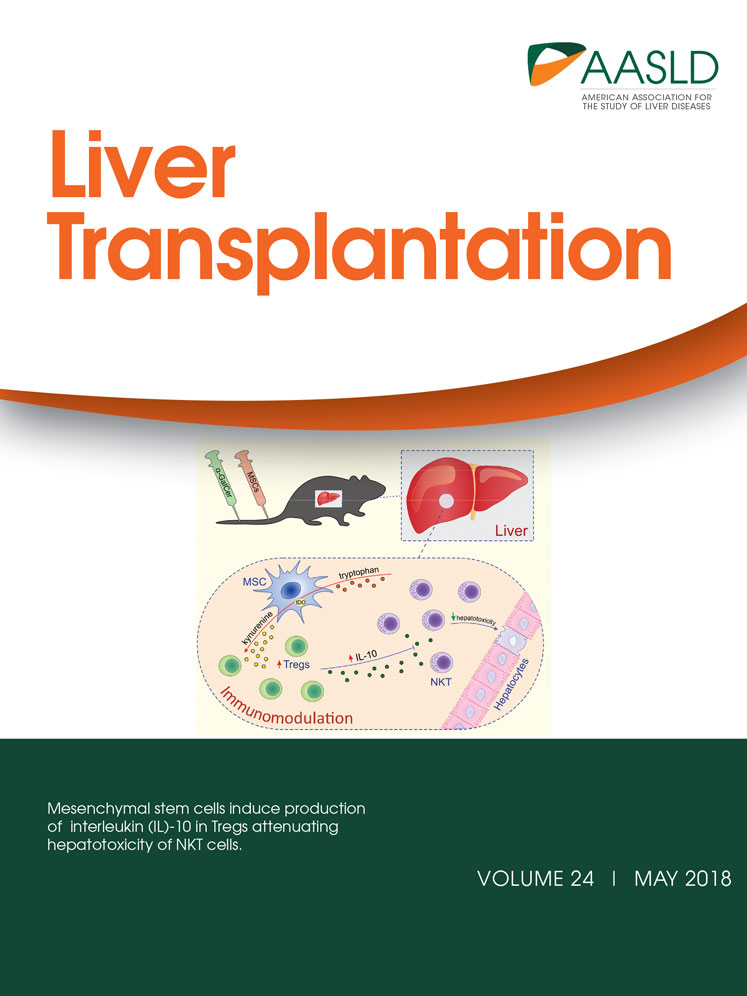
From the start of NRP, PVF, HAF, and pump flows were significantly higher among donors in the heparin group compared with the other 2. These flows remained higher in the heparin group throughout NRP (Fig. 1).
Rotational Thromboelastometry
Rotational thromboelastometry demonstrated differences between the groups in coagulation and lysis parameters prior to and during NRP (Table 1). Donors in the heparin group were adequately anticoagulated, as reflected by significantly prolonged CT and the absence of measurable clot formation on INTEM test; the effects of heparin persisted, though to a lesser extent, at the end of CA. During NRP, fibrinolytic therapy was adequate in the TPA group, as reflected by 100% lysis on HEPTEM assay. Finally, there were no differences in postreperfusion coagulation and lysis parameters in recipient pigs.
| Reference | Control | TPA | Heparin | P Values | |
|---|---|---|---|---|---|
| Pre-arrest (INTEM) | |||||
| CT, seconds | 63-212 | 151 (103-162) | 171 (160-175) | 3232 (1325-5136) | <0.05 |
| Alpha, deg | 80-85 | 81 (80-82) | 80 (80-81) | — | 0.34 |
| MCF, mm | 61-79 | 73 (71-74) | 72 (70-73) | — | 0.16 |
| LY30, % | 94-100 | 97 (95-100) | 97 (94-99) | — | 0.56 |
| LY60, % | 94-100 | 87 (86-90) | 89 (86-93) | — | 0.66 |
| ML, % | <15 | 10 (6-14) | 14 (7-19) | — | 0.52 |
| End of arrest (INTEM) | |||||
| CT, seconds | 63-212 | 131 (115-141) | 128 (119-157) | 523 (474-572) | 0.005 |
| Alpha, deg | 80-85 | 73 (67-76) | 79 (78-81) | 45 (41-49) | 0.004 |
| MCF, mm | 61-79 | 55 (50-60) | 76 (69-79) | 47 (38-56) | 0.02 |
| LY30, % | 94-100 | 100 (98-100) | 100 (99-100) | 100 | 0.31 |
| LY60, % | 94-100 | 96 (94-97) | 94 (93-96) | 97 (95-98) | 0.39 |
| ML, % | <15 | 8 (6-10) | 11 (7-13) | 7 (5-9) | 0.17 |
| NRP (HEPTEM) | |||||
| LY30, % | 94-100 | 100 (98-100) | 0 (0-2) | 93 (91-94) | 0.003 |
| LY60, % | 94-100 | 95 (90-97) | 0 | 87 (84-90) | 0.002 |
| ML, % | <15 | 13 (8-16) | 100 | 14 (10-18) | 0.007 |
| Postreperfusion (EXTEM) | |||||
| CT, seconds | 37-80 | 70 (69-81) | 76 (67-80) | 81 (67-89) | 0.72 |
| Alpha, deg | 80-86 | 75 (75-78) | 75 (74-76) | 75 (73-77) | 0.58 |
| MCF, mm | 66-81 | 67 (65-69) | 63 (63-66) | 63 (61-66) | 0.07 |
| LY30, % | 94-100 | 100 | 100 (99-100) | 100 (99-100) | 0.48 |
| LY60, % | 94-100 | 100 (98-100) | 99 (96-100) | 98 (94-100) | 0.86 |
| ML, % | <15 | 2 (1-4) | 5 (1-8) | 3 (1-9) | 0.80 |
- NOTE: Data are given as range or median (interquartile range).
TRANSPLANTATION AND POSTOPERATIVE EVOLUTION
There were no significant differences among the groups in lengths of CA (median [range]; control, 67 [65-70] minutes; TPA, 69 [66-71] minutes; and heparin, 69 [66-70] minutes), NRP (control, 61 [60-67] minutes; TPA, 65 [61-68] minutes; and heparin, 64 [63-68] minutes), cold ischemia (control, 244 [222-250] minutes; TPA, 242 [235-255] minutes; and heparin, 236 [232-243] minutes), or the anhepatic period during liver reperfusion (control, 18 [15-19] minutes; TPA, 17 [15-18] minutes; and heparin, 17 [16-18] minutes).
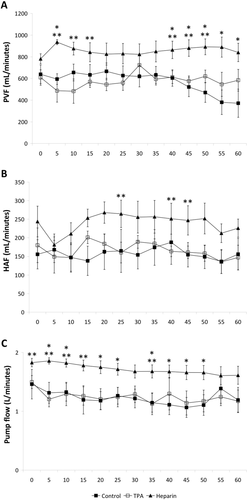
Upon reperfusion, serum AST and total bilirubin increased and QPT decreased in all of the recipients. During the follow-up period, the AST level was lower and the QPT level was higher (reflecting improved hepatic synthetic function) in the recipients of grafts from donors with pre-arrest heparinization compared with those from donors without (both the control and TPA groups; Fig. 2 A-C).
Both serum AP and GGT decreased immediately following reperfusion but then subsequently rose, with AP peaking approximately 24 hours in all 3 groups and GGT continuing to rise steadily until the end of follow-up. Although there were no differences among the groups in GGT, AP tended to be lower, at some points significantly so, in the recipients of livers treated with heparin prior to CA (Fig. 2D,E).
Five-day survival was 83% in both the control and TPA groups and 100% in the recipients of grafts treated with heparin prior to CA. One recipient in each of the control and TPA groups died between the third and fourth posttransplant days due to apparent hepatic insufficiency, with progressive deterioration of serum bilirubin and QPT preceding death.
MICROSCOPY STUDIES
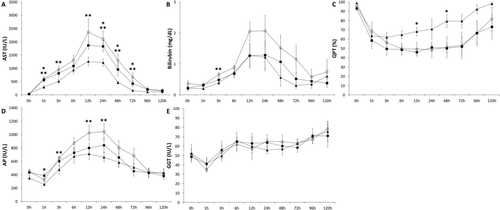
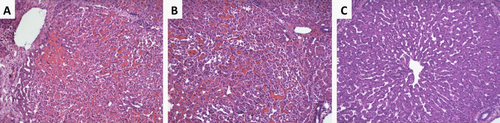
After an hour of cardiac standstill, no significant deposition of fibrin was detected in the samples, independent of treatment (pre-arrest heparinization or lack thereof). Notably, panlobular hepatocellular vacuolization was present in tissue samples taken after 1 hour of CA from livers from donors not previously treated with heparin, though this finding was relatively absent among heparin-treated grafts (Fig. 3).
Ischemia/reperfusion injury was evaluated in postreperfusion tissue biopsies. Congestion and vacuolization were observed to the same degree in all 3 groups, but less necrosis was observed among grafts treated with heparin prior to CA (Table 2; Fig. 4).
| Control | TPA | Heparin | P Value | |
|---|---|---|---|---|
| Congestion | 3.0 (2.0-3.0) | 3.0 (2.8-3.0) | 2.5 (1.0-3.0) | 0.49 |
| Vacuolization | 3.0 (2.8-3.3) | 3.0 (1.8-3.3) | 3.0 (3.0-3.3) | 0.86 |
| Necrosis | 3.0 (2.0-3.5) | 3.0 (2.3-3.5) | 1.3 (1.0-2.0) | 0.005 |
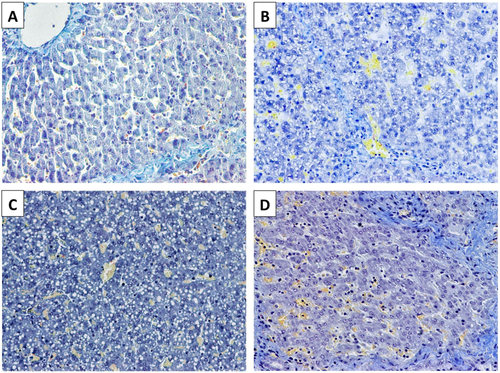
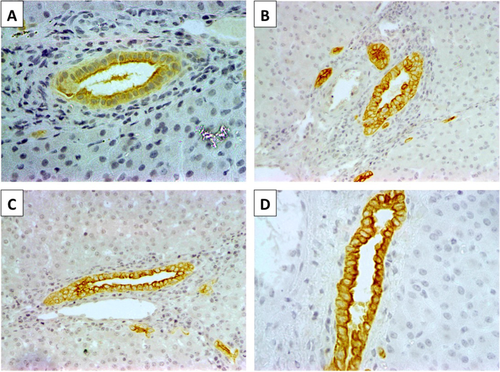
Biliary epithelial histology was evaluated with anti-CK19 immunohistochemistry. At baseline, interlobular bile ducts and ductules were lined by a uniform layer of cuboidal cells with large nuclei and scant cytoplasm. Five days after transplantation, atypical cholangiocellular proliferation/hyperplasia and partial collapse of the ductular lumen was prominent in grafts in the control and TPA groups, whereas biliary ductules were lined by a single layer of cuboidal cells with a relatively well-defined lumen among grafts in the heparin group (Fig. 5). When the peribiliary arterioles and periluminal and deep peribiliary glands were analyzed, arteriolonecrosis and peribiliary gland injury were both minimal (≤50%) in samples from the control and TPA groups, whereas no injury was observed in samples from the heparin group.
ENDOTHELIAL ACTIVATION AND INFLAMMATORY RESPONSE
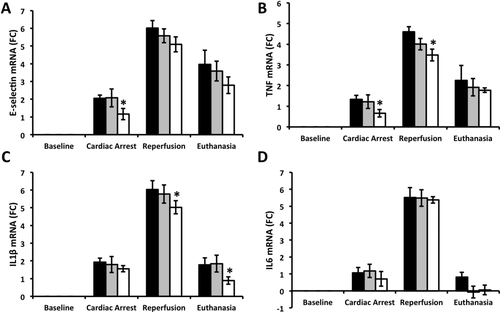
After an hour of CA, expression of E-selectin messenger RNA (mRNA), a marker of endothelial activation, was lower in grafts in the heparin group versus those without treatment (P = 0.03 and 0.05 versus control and TPA groups, respectively). Both after graft reperfusion and after 5 days of follow-up in the recipients, levels of E-selectin mRNA also tended to be lower among heparin-treated grafts followed by grafts treated with TPA during NRP and grafts in the control group.
After an hour of CA, tissue levels of TNF mRNA were lower in heparin-treated grafts (P = 0.04 for control versus heparin groups). Both TNF and IL1β mRNA levels were significantly lower among heparin-treated grafts versus controls after reperfusion (P = 0.03), and IL1β mRNA remained lower in heparin-treated grafts at the end of follow-up (P = 0.02 when compared with the control group; Fig. 6).
Discussion
The major finding of the present study is that heparin provides anti-inflammatory and other cytoprotective effects when administered prior to CA in the context of DCD, independent of its role as an anticoagulant. This finding has potential to impact clinical practice, given that heparin is not universally given prior to the withdrawal of life support in cDCD, depending on local legislation and clinical practice guidelines. As well, this study demonstrates that fibrinolytic therapy, administered in order to dissolve theoretical microthrombi formed during warm ischemia, may not be as useful as some authors have suggested.
Compared with human blood, porcine blood contains higher concentrations of fibrinogen, platelets, and plasminogen activator inhibitor-1 (PAI-1) and a lower concentration of plasminogen.19, 20 Even despite this relative hypercoagulability at baseline, there was still no fibrin clot deposition (the target of fibrinolytic therapy) in this model after an hour of cardiac standstill, regardless of whether heparin had been administered prior to arrest or not. It might be argued that a group receiving both pre-arrest heparinization and fibrinolytic therapy during NRP should have been included in this study. However, such an argument is rather counterintuitive. Adequate pre-arrest heparinization should prevent any theoretical clot from forming during the subsequent period of cardiocirculatory arrest. In this study, no microvascular clot was found, even when no therapy (neither heparin nor TPA) was given.
Given that it was performed in a porcine model, the findings of this study need to be confirmed in the clinical setting. However, there is already clinical evidence of lack of fibrin clot deposition following CA in the context of DCD. A recent clinical study from Rotterdam evaluated 282 sections from 94 biopsies from discarded human cDCD liver grafts (n = 16) triple-stained to detect the presence of microthrombi. Microvascular clot with fibrin was only found in 1%-3% of the sections evaluated. Furthermore, when the authors evaluated cDCD and donation after brain death (DBD) grafts from an earlier era, microthrombi were found at rates of 3% and 11%, respectively, and there was no association between the presence of microthrombi and the subsequent development of ITBL.21 Other authors have similarly studied histopathological changes associated with the appearance of ITBL after both DBD and cDCD liver transplantation in the clinical setting and have also described low rates of microvascular thrombi and no significant association between the presence of microthrombi and the development of ITBL.17, 18
Several years ago, Porte and Clavien highlighted the fact that platelet activation is reduced and coagulation altered early after death, with less stable clot formation and marked activation of the fibrinolytic system.22 More recently, our group used ROTEM to show that potential uDCD donors universally suffer hyperfibrinolysis at the moment death is declared.23 Other groups have used thromboelastometry to study patients suffering CA, and it appears that the incidence of hyperfibrinolysis increases in relation to the length of arrest and warm ischemia.24 When organs and tissues suffer progressive ischemia, thrombomodulin, an integral membrane protein expressed on the surface of endothelial cells, is induced. Thrombomodulin serves as a cofactor for thrombin and converts it from a procoagulant to an anticoagulant enzyme, diverting it from fibrin generation to the activation of protein C.25, 26 Activated protein C consumes PAI-1, thereby reducing the inhibition of TPA and accelerating the conversion of plasminogen to plasmin.27-29 Simultaneously, hypoxia and a secondary systemic rise in catecholamines cause the release of TPA from endothelial cells, leading to excessive fibrinolysis.30, 31
In the present model, CA is sudden, as is the case in human uDCD. In uDCD, given the abrupt and unexpected nature of arrest, the donor and liver undergo a very prolonged period of warm ischemia, and the risk for the development of ischemic biliary complications in the posttransplant period is very high if aggressive donor recovery and maintenance and strict organ selection criteria are not employed.5, 32, 33 Although this model lacks the “agonal phase” seen in the other clinical scenario of cDCD, it is unlikely that the addition of an agonal phase would have changed anything with regard to fibrin deposition (or, in this case, the lack thereof). It has been consistently seen that acutely critically ill patients suffering from a variety of conditions (eg, trauma, sepsis, myocardial infarction, or post-CA syndrome) present severe hemostatic alterations characterized by extreme hypocoagulability and hyperfibrinolysis. This condition, which has recently been denominated shock-induced endotheliopathy (“SHINE”), appears to be a compensatory mechanism to improve perfusion when tissue and cellular oxygenation is significantly reduced (ie, when the patient is in a state of shock, regardless of the origin).34 The coagulation profiles of cDCD donors have not been specifically evaluated in the context of any clinical study. (We would encourage anyone with the means to do so; in our setting, however, all potential cDCD donors are systematically heparinized prior to the withdrawal of life support.) However, given that the agonal phase is also characterized by critical cellular and tissue hypoxia, it is very likely that it, too, provokes the same systemic changes of hypocoagulability and hyperfibrinolysis seen in the aforementioned clinical scenarios.
After 1 hour of warm ischemia, hepatocellular vacuolization, which was prominent among livers in the control and TPA groups (untreated up until that point), was significantly less among livers treated with heparin prior to arrest. Hepatocellular vacuolization has been observed in livers exposed to prolonged warm ischemia,35 and the extent of vacuolization has been correlated with the degree of hepatocellular damage.36 Theories as to the etiology of hepatocellular vacuolization include influx of plasma during cellular anoxia,37 lysosomal degradation (ie, autophagic vacuoles),38 or internalization of apical membranes due to adenosine triphosphate depletion.39 Although the exact mechanism remains to be elucidated, it is possible binding of heparin, which is known to have direct anti-inflammatory and cytoprotective effects and to reduce the expression of cellular adhesion molecules,40-42 resulted in cell membrane stabilization during warm ischemia. These collateral effects of heparin could also be observed in liver tissue gene levels of E-selectin, TNF, IL1β, and IL6, which were lowest in this study among grafts arising from donors treated with heparin prior to CA. Furthermore, it is plausible that this protective effect on the endothelium resulted in improved flows during NRP and, consequentially, improvements in postreperfusion liver biochemical, functional, and inflammatory parameters measured over the course of follow-up.
Anti-CK19 immunostaining was used to evaluate biliary histological changes in this study. Bile ductules are very reactive anatomical elements of the liver.43 Proliferative/hyperplasic ductular changes were observed in samples taken 5 days after transplantation from grafts in the control and TPA groups, whereas these changes were relatively absent in grafts in the heparin group. In accordance with the previous commentary, these findings are reflective of, if anything, the adequacy of perfusion during NRP. Mounting clinical evidence suggests that NRP has a significant positive impact on biliary preservation in the setting of DCD liver transplantation because it limits the length of warm ischemia, restores cellular energy stores, and reconditions the graft prior to cold perfusion and storage.11, 12 There are now several single-center series that describe excellent posttransplantation survival rates and virtually no ITBL using DCD livers recovered with postmortem NRP.44-47 At our own center, postmortem NRP has been used to recover livers from cDCD donors.13 Fifteen livers recovered in this fashion have been transplanted since 2014, with 92% 1-year graft survival and not a single case of ITBL.
Apart from the fact that it is not beneficial, the administration of a fibrinolytic drug to a graft, which may have ongoing effects even after washout due to binding to the vascular endothelium,48 is an intervention that could pose substantial risk for the development of uncontrollable hemorrhage in the recipient. This issue is particularly relevant in the setting of DCD liver transplantation, where postreperfusion coagulopathy is not uncommon.49, 50 Nonetheless, there are clinical reports that allege benefit for the use of fibrinolytic therapy in this setting. The Cleveland Clinic Group described a series of 22 transplants performed with cDCD grafts treated with TPA injected into the donor hepatic artery on the back table, with or without heparin administered systemically prior to ventilator withdrawal. There was no control group in this study, and ITBL arose postoperatively in 1 (9%) patient, including 1 in which heparin had been given prior to withdrawal. Notably, 64% of recipients had excessive postreperfusion bleeding.7 Seal et al. subsequently published a retrospective review of cDCD liver transplants performed at 2 North American centers wherein a small dose of TPA was administered directly into the hepatic artery prior to completing the portal anastomosis in 85 of 113 cDCD recipients transplanted during a 7-year period. The authors described a lower rate of biliary strictures in the TPA-treated recipients (17% versus 33%; P = 0.07).8 It is important to note, however, that patients were not randomized. The authors compared recipients of livers treated with TPA with controls from an earlier era. As well, donors were fully heparinized prior to withdrawal. One of the same 2 centers recently published a retrospective analysis of 138 cDCD liver transplants performed in which the last 100 recipients received low-dose TPA and verapamil injected directly into the hepatic artery immediately following graft reperfusion in the recipient. Donors in both eras were heparinized prior to withdrawal, and the rates of ITBL in both eras were low (3%-5%) and not significantly different.10 Also, the Indiana University Group compared cDCD livers treated with TPA added to the aortic flush and subsequently directly injected into the hepatic artery (n = 30) with a historic group of controls from an earlier era (n = 61). They claimed the use of TPA significantly reduced the rates of both anastomotic and nonanastomotic biliary strictures, though donor age was greater and the warm and cold ischemia times significantly longer in non-TPA grafts.9 In contrast, a study from Leiden compared recipients of livers treated with urokinase during back-table surgery (n = 63: 46 DBD and 17 cDCD) with a group of controls from an earlier era (n = 122: 94 DBD and 28 cDCD). The control group had lower body mass index but longer cold ischemia times, but rates of ITBL were exactly the same in both groups (11% and 39% among DBD and cDCD recipients, respectively, in the control group versus 10% and 41% among DBD and cDCD recipients, respectively, in the study group).51
In summary, this experimental study suggests that fibrinolytic therapy does not play a role in improving liver grafts arising through the DCD process due to lack of fibrin clot deposition during CA. Although it may not be needed for its anticoagulant properties, heparin does offer anti-inflammatory and other cytoprotective effects that may be useful to help improve liver graft quality when administered prior to withdrawal of life support/CA in the context of cDCD.
Acknowledgments
The authors would like to thank Dr. Marc Net and Institut Georges Lopez for providing the Institut Georges Lopez 1 solution used in these studies.