Exogenous vascular endothelial growth factor delivery prior to endothelial precursor cell transplantation in orthotopic liver transplantation–induced hepatic ischemia/reperfusion injury
Jinzheng Li and Jianping Gong conceived and designed the study. Ding Cao and Menghao Wang performed the experiments. Junhua Gong and Sidong Wei analyzed the data. Ding Cao drafted the manuscript.
This work was supported by the National Natural Science Foundation of China (grant numbers 81370577, 81200329, 81300364, and 81370580). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abstract
Vascular endothelial growth factor (VEGF) promotes angiogenesis in vivo. We hypothesized that exogenous delivery of VEGF prior to bone marrow–derived endothelial precursor cell (EPC) transplantation may improve orthotopic liver transplantation (OLT)–induced hepatic ischemia/reperfusion injury (HIRI). OLT between Sprague Dawley donor rats and inbred LEW Wistar recipient rats was performed in 6 experimental groups to comparatively assess the effects of the VEGF gene: an untreated normal control group, a surgical control group, a liposomal control group, a VEGF group receiving only the liposome-encapsulated VEGF plasmid, an EPC group receiving only EPCs, and an EPC+VEGF group receiving the liposome-encapsulated VEGF plasmid followed by EPCs. VEGF plasmid delivery to liver tissue, endogenous VEGF, and vascular endothelial growth factor receptor (VEGFR) expression, liver transaminase levels, hepatocellular injury levels, apoptosis, apoptotic biomarkers, hepatotrophic mitogens, angiogenesis, and nitric oxide synthase (NOS) activity were assayed after OLT. Exogenous VEGF gene delivery prior to EPC transplantation significantly increased endogenous VEGF and VEGFR expression, significantly reduced liver transaminase levels, significantly reduced hepatocellular injury levels, significantly reduced hepatic apoptosis levels, and significantly reduced several apoptotic biomarkers (ie, B cell lymphoma 2–associated X protein/B cell lymphoma 2 ratio, caspase 3 activity, and heat shock protein 70 expression) in post-OLT–induced HIRI. Moreover, VEGF gene delivery prior to EPC transplantation significantly increased hepatotrophic mitogen expression (ie, epidermal growth factor, heparin-binding epidermal growth factor–like growth factor, hepatocyte growth factor, and transforming growth factor α), angiogenesis, and NOS activity in post-OLT–induced HIRI. In conclusion, exogenous liposomal delivery of the VEGF gene prior to bone marrow–derived EPC transplantation may be an effective strategy in decreasing OLT-induced HIRI. Liver Transplantation 23 804–812 2017 AASLD.
Abbreviations
-
- ALT
-
- alanine aminotransferase
-
- ASC
-
- apoptosis-associated speck-like protein containing a caspase recruitment domain
-
- AST
-
- aspartate aminotransferase
-
- BAX
-
- B cell lymphoma 2–associated X protein
-
- BCL2
-
- B cell lymphoma 2
-
- CO
-
- carbon monoxide
-
- Dil-ac-LDL+
-
- dil-acetylated low-density lipoprotein positive
-
- DOTAP
-
- 1,2-dioleoyl-3-trimethylammonium-propane
-
- EGF
-
- epidermal growth factor
-
- eNOS
-
- endothelial nitric oxide synthase
-
- EPC
-
- endothelial precursor cell
-
- FLk-1
-
- fetal liver kinase-1
-
- Flt-1
-
- fms related tyrosine kinase 1
-
- H & E
-
- hematoxylin-eosin
-
- HB-EGF
-
- heparin-binding epidermal growth factor–like growth factor
-
- HGF
-
- hepatocyte growth factor
-
- HIRI
-
- hepatic ischemia/reperfusion injury
-
- HO-1
-
- heme oxygenase 1
-
- HSP70
-
- heat shock protein 70
-
- iNOS
-
- inducible nitric oxide synthase
-
- KDR
-
- kinase insert domain receptor
-
- LCON
-
- liposomal control
-
- LEW
-
- Lewis rats
-
- LSEC
-
- liver sinusoidal endothelial cell
-
- MTT
-
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
-
- NCON
-
- normal control
-
- NOS
-
- nitric oxide synthase
-
- OLT
-
- orthotopic liver transplantation
-
- RFU
-
- relative fluorescence units
-
- SCON
-
- surgical control
-
- TGF-α
-
- transforming growth factor α
-
- TUNEL
-
- terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling
-
- UEA-1+
-
- Ulex europaeus agglutinin 1 positive
-
- VEGF
-
- vascular endothelial growth factor
-
- VEGFR
-
- vascular endothelial growth factor receptor
Orthotopic liver transplantation (OLT) is a well-established surgical intervention for acute liver failure or end-stage liver disease.1 Unfortunately, persistent liver dysfunction from OLT-induced hepatic ischemia/reperfusion injury (HIRI) remains a problematic issue.1 OLT-induced HIRI results from the hypoxic insult produced by the disrupted blood flow followed by the subsequent reinitiation of blood flow that produces injurious immune and inflammatory responses.2 This OLT-induced HIRI is a clinically significant disease process leading to microangiopathy, abnormal tissue perfusion, and eventual liver dysfunction.3
To address the challenge of OLT-induced HIRI, exogenous transplantation of bone marrow–derived endothelial precursor cells (EPCs) has been shown to promote local angiogenesis through endothelial differentiation and release of angiogenic factors.4 Moreover, exogenous transplantation of bone marrow–derived EPCs has been shown to significantly improve liver regeneration and promote resolution of liver fibrosis.4, 5 Additionally, growth factors have been shown to play important roles in liver regeneration after HIRI.6 Therefore, combining the exogenous transplantation of bone marrow–derived EPCs with exogenous delivery of growth factors may provide a means by which to improve the in vivo function of EPCs in treating OLT-induced HIRI. Specifically, vascular endothelial growth factor (VEGF) has been identified as an important growth factor in promoting angiogenesis and has been shown to stimulate hepatocyte growth factor (HGF) expression, which functions to initiate liver regeneration.7
On the basis of this evidence, we hypothesized that exogenous delivery of the VEGF gene prior to bone marrow–derived EPC transplantation may improve OLT-induced HIRI. In this study, we evaluated VEGF gene delivery as an adjunctive therapy to bone marrow–derived EPC transplantation in a rodent model of OLT-induced HIRI.
Materials and Methods
ETHICS STATEMENT
The experimental protocols of this study were approved by the Ethics Committee of the Second Affiliated Hospital of Chongqing Medical University (Chongqing, China). All animal procedures were carried out in accordance with the recommendations provided in the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD). All surgical procedures were performed under ether anesthesia, and all efforts were made to minimize animal suffering.
- Isolation and cultivation of EPCs from rat bone marrow.
- Identification of EPCs by fluorescent staining.
- The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay to assess EPC viability.
- VEGF plasmid construction and amplification in E. coli (VEGF plasmid construction was performed as previously described).8
- Preparation of VEGF Plasmid: Liposome complexes (preparation of the cationic DOTAP: Chol liposomes was performed as previously described).9
- OLT (an improved Kamada's 2-cuff method was applied for OLT between SD donor rats and Lewis rats (LEW) Wistar recipient rats as previously described with minor modifications).10
- Liver transaminase assays (hepatic injury was assessed via liver transaminase activity as previously described).11
- Western blotting (Western blotting was performed as previously described with minor modifications).12
- Staining and quantification of apoptosis, hepatocellular injury, and vascularization (terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling [TUNEL] and hematoxylin-eosin [H & E] staining and analysis was performed as previously described with minor modifications).13-15
- Assessment of nitric oxide synthase (NOS) and caspase 3 activity (NOS and caspase 3 activity were assayed as previously described).16, 17
- Statistical analysis (statistical analyses were performed with SPSS, version 19 [IBM, Armonk, NY]).
Results
Prior to transplantation, EPCs were positively identified as dil-acetylated low-density lipoprotein positive (Dil-ac-LDL+)/Ulex europaeus agglutinin 1 positive (UEA-1+) cells (Supporting Fig. 1). An MTT assay performed to confirm EPC viability prior to transplantation revealed robust EPC viability over 72 hours in vitro (Supporting Fig. 2). Post-VEGF gene delivery, we validated exogenous VEGF plasmid delivery to the liver tissue of the VEGF-treated groups (ie, the VEGF and EPC+VEGF groups) by fluorescent microscopy at 3 hours after reperfusion (Supporting Fig. 3).
Next, we assessed endogenous hepatic expression of VEGF, vascular endothelial growth factor receptor (VEGFR)–1, and VEGFR-2 at 3 hours and 6 hours after reperfusion. The VEGF, EPC, and EPC+VEGF groups displayed significantly elevated VEGF, VEGFR-1, and VEGFR-2 expression relative to liposomal controls (LCONs; Supporting Fig. 4; Supporting Results). Moreover, the EPC+VEGF group showed significantly higher VEGF, VEGFR-1, and VEGFR-2 expression relative to both the VEGF and EPC groups (Supporting Fig. 4).
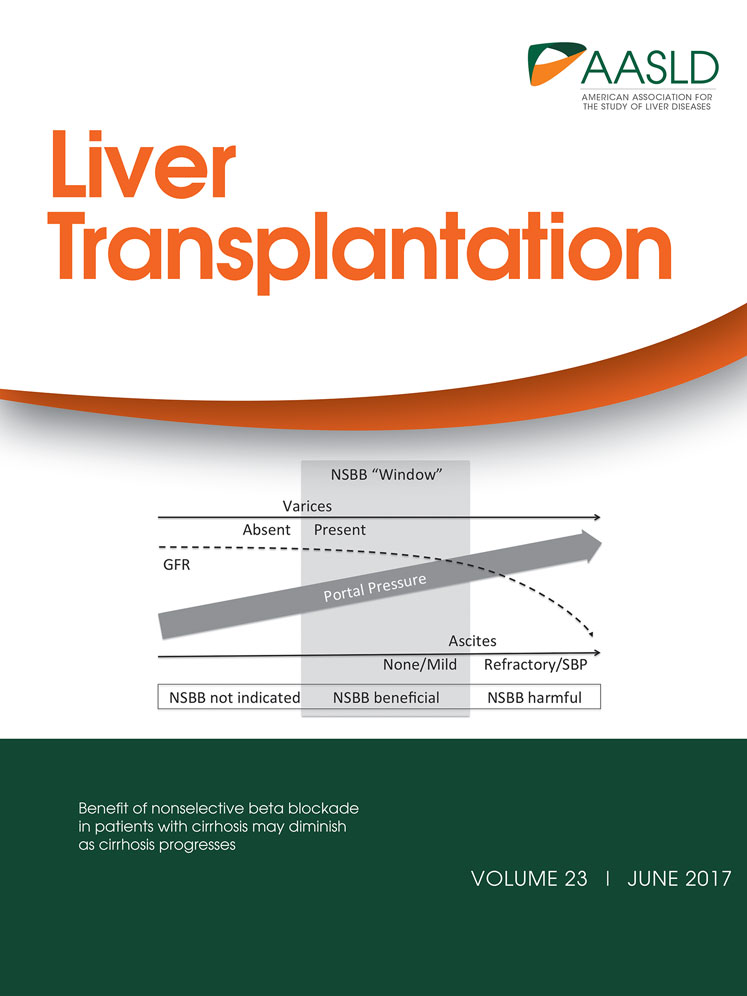
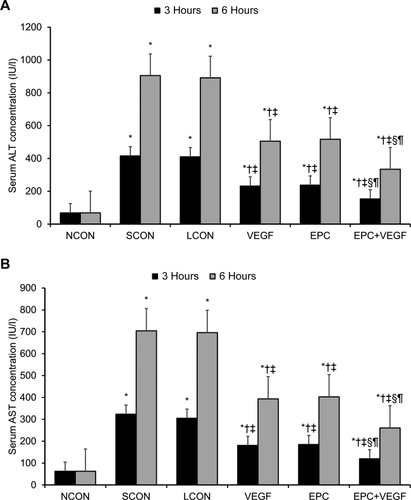
Next, we assessed gross levels of hepatic injury by liver transaminase assays at 3 hours and 6 hours after reperfusion. Relative to LCONs, the VEGF, EPC, and EPC+VEGF groups displayed significantly lower alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels (Fig. 1A,B; Supporting Results). Moreover, the EPC+VEGF group displayed significantly lower ALT and AST levels relative to the VEGF and EPC groups (Fig. 1A,B; Supporting Results). Next, we assessed hepatocellular injury levels by Suzuki scoring at 3 hours and 6 hours after reperfusion. Relative to LCONs, the VEGF, EPC, and EPC+VEGF groups displayed significantly lower Suzuki scores (Fig. 2A,B; Supporting Information). Moreover, the EPC+VEGF group showed significantly lower Suzuki scores relative to the EPC and VEGF groups (Fig. 2A,B; Supporting Results).
VEGF gene delivery prior to EPC transplantation reduces gross hepatic injury.
Liver transaminase assays of (A) ALT and (B) AST were applied to assess gross hepatic injury at 3 hours and 6 hours after reperfusion. *P < 0.05 relative to NCON group; †P < 0.05 relative to SCON group; ‡P < 0.05 relative to LCON group; §P < 0.05 relative to VEGF group; ¶P < 0.05 relative to EPC group.
VEGF gene delivery prior to EPC transplantation reduces hepatocellular injury and apoptosis. (A) Representative HE-stained images of liver tissue at 3 hours and 6 hours after reperfusion. Hepatocellular injury was evidenced by hepatocyte necrosis, ballooning degeneration, and sinusoidal congestion. Scale bar = 50 µm. (B) Tabulation of Suzuki scores across the experimental groups as an indicator of hepatocellular injury. (C) Representative TUNEL-stained images of liver tissue at 3 hours and 6 hours after reperfusion. Apoptotic cells displayed cell shrinkage, chromatin condensation, brown-colored nuclei, and positive-staining apoptotic bodies. Scale bar = 50 µm. (D) Tabulation of the TUNEL + cell percentage across the experimental groups as an indicator of apoptosis levels. *P < 0.05 relative to NCON group; †P < 0.05 relative to SCON group; ‡P < 0.05 relative to LCON group; §P < 0.05 relative to VEGF group; ¶P < 0.05 relative to EPC group.
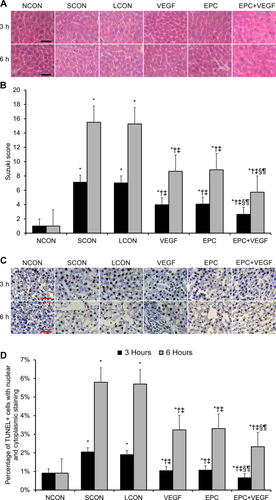
Next, we assessed hepatic apoptosis levels by TUNEL staining at 3 hours and 6 hours after reperfusion. Relative to LCONs, the VEGF, EPC, and EPC+VEGF groups displayed significantly lower apoptosis levels (Fig. 2C,D; Supporting Results). Moreover, the EPC+VEGF group showed significantly lower apoptosis levels relative to the EPC and VEGF groups (Fig. 2C,D; Supporting Results). Having shown evidence of significant changes in apoptosis levels between the experimental groups, we next assayed several key biomarkers of apoptosis at 3 hours and 6 hours after reperfusion. First, relative to LCONs, the VEGF, EPC, and EPC+VEGF groups displayed significantly lower B cell lymphoma 2–associated X protein (BAX)/B cell lymphoma 2 (BCL2) ratios—a p53-independent predictor of hepatic apoptosis activity (Fig. 3A-C; Supporting Results).18 Moreover, the BAX/BCL2 ratio was significantly lower in the EPC+VEGF group relative to the EPC and VEGF groups (Fig. 3A-C; Supporting Results). These observed effects upon the BAX/BCL2 ratio were primarily driven through changes in BAX as opposed to BCL2 (Fig. 3A-C). Second, relative to LCONs, the VEGF, EPC, and EPC+VEGF groups displayed a significantly lower activity of caspase 3—a proapoptotic “effector” caspase in liver tissue (Fig. 3D; Supporting Results).19 Moreover, caspase 3 activity was significantly lower in the EPC+VEGF group relative to the VEGF and EPC groups at 3 hours after reperfusion (Fig. 3D; Supporting Results). Third, relative to LCONs, the VEGF, EPC, and EPC+VEGF groups displayed a significantly lower expression of heme oxygenase 1 (HO-1)—a repressor of apoptosis in hepatic tissue (Fig. 3E; Supporting Results).20 However, HO-1 expression was not significantly different across the EPC+VEGF, EPC, and VEGF groups (Fig. 3E; Supporting Results). Fourth, relative to LCONs, the VEGF, EPC, and EPC+VEGF groups displayed a significantly lower expression of heat shock protein 70 (HSP70)—a chaperone protein that is up-regulated in response to stress-induced apoptosis in the liver (Fig. 3F; Supporting Results).21 Moreover, HSP70 expression was significantly lower in the EPC+VEGF group relative to the EPC and VEGF groups at 3 hours after reperfusion (Fig. 3F; Supporting Results).
VEGF gene delivery prior to EPC transplantation reduces apoptotic biomarker expression. Western blotting and a caspase activity assay were applied to assess several key molecular biomarkers of apoptosis at 3 hours and 6 hours after reperfusion. (A) BAX expression. (B) BCL2 expression. (C) BAX/BCL2 ratio. (D) Caspase 3 activity. (E) HO-1 expression. (F) HSP70 expression. *P < 0.05 relative to NCON group; †P < 0.05 relative to SCON group; ‡P < 0.05 relative to LCON group; §P < 0.05 relative to VEGF group; ¶P < 0.05 relative to EPC group.
Next, we assessed the expression of 4 key hepatotrophic mitogens (ie, epidermal growth factor [EGF], heparin-binding epidermal growth factor–like growth factor [HB-EGF], HGF, and transforming growth factor α [TGF-α]) at 3 hours and 6 hours after reperfusion.22-24 Relative to LCONs, the VEGF, EPC, and EPC+VEGF groups displayed significantly higher expression of EGF, HB-EGF, HGF, and TGF-α (Fig. 4A-D, Supporting Results). Moreover, EGF, HB-EGF, and HGF expression were significantly higher in the EPC+VEGF group relative to the EPC and VEGF groups at 3 hours after reperfusion (Fig. 4A-C; Supporting Results), whereas TGF-α expression was significantly higher in the EPC+VEGF group relative to the EPC and VEGF groups at 6 hours after reperfusion (Fig. 4D, Supporting Results).
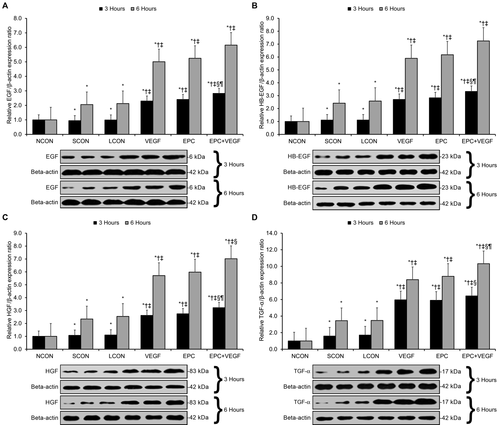
VEGF gene delivery prior to EPC transplantation promotes hepatotrophic mitogen expression. Western blotting was applied to assess the protein expression of several key hepatotrophic mitogens at 3 hours and 6 hours after reperfusion. (A) EGF expression. (B) HB-EGF expression. (C) HGF expression. (D) TGF-α expression. *P < 0.05 relative to NCON group; †P < 0.05 relative to SCON group; ‡P < 0.05 relative to LCON group; §P < 0.05 relative to VEGF group; ¶P < 0.05 relative to EPC group.
Finally, we comparatively assessed VEGF-induced angiogenesis levels across the experimental groups.25 By microscopic examination, all surgical groups showed local and sinusoidal bleeding (Fig. 5A). Qualitatively, the EPC+VEGF group appeared to show greater liver sinusoidal endothelial cell (LSEC) proliferation and greater intact blood vessel wall formation relative to the EPC and VEGF groups (Fig. 5A). Quantitatively, relative to LCONs, the VEGF, EPC, and EPC+VEGF groups displayed a significantly larger number of vascular sections per field (Fig. 5B; Supporting Results). Moreover, the EPC+VEGF group displayed a significantly larger number of vascular sections per field relative to the EPC and VEGF groups (Fig. 5B; Supporting Results). With respect to endothelial nitric oxide synthase (eNOS) activity, relative to LCONs, the VEGF and EPC+VEGF groups displayed a significantly higher eNOS activity (Fig. 5C; Supporting Results). Moreover, the EPC+VEGF group displayed significantly higher eNOS activity relative to the EPC and VEGF groups at 3 hours after reperfusion (Fig. 5C; Supporting Results). With respect to inducible nitric oxide synthase (iNOS) activity, relative to LCONs, the VEGF, EPC, and EPC+VEGF groups displayed a significantly higher iNOS activity (Fig. 5D; Supporting Results). Moreover, the EPC+VEGF group displayed significantly higher iNOS activity relative to the EPC and VEGF groups at 3 hours after reperfusion (Fig. 5D, Supporting Results).
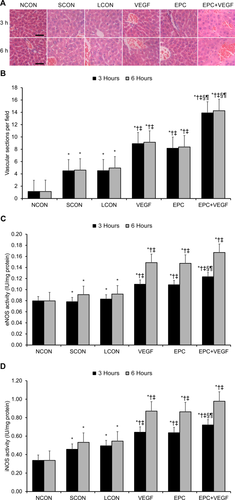
VEGF gene delivery prior to EPC transplantation promotes angiogenesis and NOS activity. (A) Representative H & E-stained images of liver tissue at 3 hours and 6 hours after reperfusion. Scale bar = 50 µm. (B) Vascular sections per field 3 hours and 6 hours after reperfusion. (C) eNOS activity at 3 hours and 6 hours after reperfusion. (D) iNOS activity at 3 hours and 6 hours after reperfusion. *P < 0.05 relative to NCON group; †P < 0.05 relative to SCON group; ‡P < 0.05 relative to LCON group; §P < 0.05 relative to VEGF group; ¶P < 0.05 relative to EPC group.
Discussion
Here, we evaluated exogenous VEGF gene delivery as an adjunctive therapy to bone marrow–derived EPC transplantation in a rodent model of OLT-induced HIRI. First, we found that VEGF gene delivery prior to EPC transplantation significantly enhances endogenous hepatic VEGF and VEGFR expression in post-OLT–induced HIRI. Second, we found that VEGF gene delivery prior to EPC transplantation significantly reduces hepatocellular injury in post-OLT–induced HIRI. Third, we found that VEGF gene delivery prior to EPC transplantation significantly reduces hepatic apoptosis levels as well as several key apoptosis biomarkers in post-OLT–induced HIRI. Fourth, we found that VEGF gene delivery prior to EPC transplantation significantly increases the expression of several potent hepatotrophic mitogens in post-OLT–induced HIRI. Finally, we found that VEGF gene delivery prior to EPC transplantation significantly increases angiogenesis and NOS activity in post-OLT–induced HIRI. On the basis of this evidence, exogenous liposomal delivery of the VEGF gene prior to bone marrow–derived EPC transplantation may be an effective strategy in decreasing OLT-induced HIRI.
VEGF is a potent endothelial growth factor that has been shown to be expressed at low baseline levels in quiescent normal hepatocytes.26 Following liver injury, both hepatocytes and HSCs have been shown to quickly up-regulate their VEGF secretion in response to cytokines as well as hypoxia in order to support hepatic recovery.26 VEGF acts on its target cells via 2 key tyrosine kinase receptors named VEGFR-1 (alternatively fms related tyrosine kinase 1 [Flt-1]) and VEGFR2 (alternatively fetal liver kinase-1 [Flk-1] and kinase insert domain receptor [KDR]).27, 28 Because increased VEGF expression has been shown to be linked to both VEGFR-1 up-regulation on hepatocytes as well as VEGFR-1 and VEGFR-2 up-regulation on LSECs,26 here we successfully confirmed the effective liposomal delivery of the VEGF gene to the target liver tissue of the VEGF-treated groups. Notably, although the VEGF and EPC+VEGF groups received identical doses of the VEGF plasmid, the EPC+VEGF group displayed significantly higher endogenous VEGF, VEGFR-1, and VEGFR-2 expression over the EPC group. This finding reveals that VEGF gene delivery prior to EPC transplantation enhances endogenous hepatic VEGF and VEGFR expression in post-OLT–induced HIRI.
The increased levels of hepatocellular apoptosis observed in HIRI are driven through several interrelated mechanisms.29 For instance, the apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) associates with BAX to activate the proapoptotic p53/BAX pathway.30 ASC activity also decreases antiapoptotic BCL2 expression while increasing proapoptotic caspase 3 expression.30 In addition, HIRI also activates expression of HO-1 (also known as HSP32), which provides protection against HIRI through carbon monoxide (CO) generation.31 Mechanistically, HO-1 promotes the expression of several antiapoptotic proteins through CO generation, including BCL2 and HSP70.32, 33 Consistent with these findings, we found that OLT-induced HIRI increased hepatic apoptosis levels, the BAX/BCL2 ratio, caspase 3 activity, HO-1 expression, and HSP70 expression. Notably, VEGF gene delivery prior to EPC transplantation was able to reduce hepatic apoptosis levels, the BAX/BCL2 ratio, caspase 3 activity, and HSP70 expression in post-OLT–induced HIRI. Although VEGF has been shown to induce BCL2 expression in endothelial cells,34 further investigation is needed on the mechanism(s) by which VEGF modulates the expression of other apoptotic mediators in post-OLT–induced HIRI.
Quiescent normal hepatocytes display a wide array of receptors for growth factors.35 Despite the expression of several growth factor receptors, evidence has defined a limited set of growth factors that actually function as direct hepatotrophic mitogens both in vitro and in vivo; these factors are the key EGFR ligands (ie, EGF, HB-EGF, and TGF-α) as well as HGF.35 Therefore, here we only analyzed the expression of these 4 mitogens. VEGF stimulates hepatocyte proliferation through VEGFR-1–induced HGF up-regulation.36, 37 Specifically, VEGF's binding to LSECs triggers the release of pro-HGF, which is then cleaved to form HGF.31, 37 HGF then activates the release of TGF-α and other downstream signals that promote cell-cycle transition.31 Moreover, VEGF's binding to LSECs also triggers the release of HB-EGF through an overlapping VEGFR-1/2–based mechanism.37 Although VEGF has not been shown to directly up-regulate EGF transcript expression through either VEGFR-1 or VEGFR-2,37 VEGFR-1–expressing macrophages secrete EGF and increasing VEGFR-1 signaling has been shown to up-regulate EGF expression in VEGF-stimulated macrophages.27 Consistent with this evidence, we observed that VEGF gene delivery prior to EPC transplantation was able to increase the expression of all 4 hepatotrophic factors (ie, EGF, HB-EGF, HGF, and TGF-α) in post-OLT–induced HIRI.
VEGF has been well established as an angiogenic factor, as it promotes endothelial cell proliferation as well as the expression of various proteases that enable new blood vessel formation.26 As VEGFR-2 activation by VEGF promotes LSEC proliferation, normal VEGF-induced angiogenesis is held to be mediated by VEGFR-2, whereas VEGFR-1 activation can lead to dysfunctional angiogenesis.26, 27 Moreover, VEGFR-2 activation (as opposed to VEGFR-1 activation) by VEGF has been shown to up-regulate the proangiogenic NO-producing enzymes eNOS and iNOS.38 Consistent with this evidence, VEGF gene delivery prior to EPC transplantation increased vascular formation and NOS activity in post-OLT–induced HIRI.
In conclusion, exogenous liposomal delivery of the VEGF gene prior to bone marrow–derived EPC transplantation may be an effective strategy in decreasing OLT-induced HIRI. Further research with various VEGF delivery models in combination with EPC transplantation in other animal models of OLT-induced HIRI are warranted.