Epstein-Barr virus–associated lymphoepithelial carcinoma after pediatric liver transplant
These studies were supported by the National Institutes of Health (UO1 Al104342) and the Roche Organ Transplant Research Foundation. Additional support was provided by the Ernest and Amelia Gallo Endowed Postdoctoral Fellowship as well as the Child Health Research Institute and the Stanford CTSA (UL1 TR001085).
Abbreviations
-
- CT
-
- computed tomography
-
- EBER
-
- Epstein-Barr virus-encoded small RNA
-
- EBNA
-
- Epstein-Barr nuclear antigen
-
- EBV
-
- Epstein-Barr virus
-
- LEC
-
- lymphoepithelial carcinoma
-
- LFT
-
- liver function test
-
- LMP1
-
- latent membrane protein 1
-
- MRI
-
- magnetic resonance imaging
-
- NIH
-
- National Institutes of Health
-
- PCR
-
- polymerase chain reaction
-
- PTLD
-
- posttransplant lymphoproliferative disorder
-
- UPGMA
-
- unweighted pair group method with arithmetic mean
De novo malignancy is one of the feared longterm complications following organ transplantation. For the pediatric population, the most common malignancy following liver transplantation is posttransplant lymphoproliferative disorder (PTLD), which has a reported incidence of 1% to 20%. Most occurrences of PTLD are associated with Epstein-Barr virus (EBV) infection of B lymphocytes. Pediatric transplant recipients, who are usually EBV-naïve and immunosuppressed, are at much higher risk of developing PTLD following an acute EBV infection. However, transplant recipients can also develop other rarer forms of EBV-associated malignancies, such as smooth muscle tumors,1 which are poorly described in the literature. Here, we present the case of a pediatric liver recipient who developed an acute illness that was initially suspicious for PTLD, until the surprising diagnosis of lymphoepithelial carcinoma (LEC) was made. LEC is already extremely rare in the pediatric population. To our knowledge, this is the first described case of this EBV-associated carcinoma in the posttransplant setting.
Case Report
An 11-month-old girl of Asian ethnicity underwent a liver transplant from a deceased donor for biliary hypoplasia. The child was EBV-negative at the time of transplantation. Donor EBV serology is not available. She was not given any induction immunosuppression. Four months later, the child developed a maculopapular rash and fevers and was found to have an acute EBV infection by serology and polymerase chain reaction (PCR). An extensive workup for PTLD was negative, and the infection resolved. For the next 18 years, the patient was in good general health during her annual follow-up visits. Her maintenance immunosuppression consisted of low-dose tacrolimus monotherapy (1-mg bid), with consistently subtherapeutic serum levels (below 5 ng/mL). She did not have any history of graft rejection. She also had a consistently low EBV burden, determined by quantitative PCR, except for 1 flare-up at age 15.
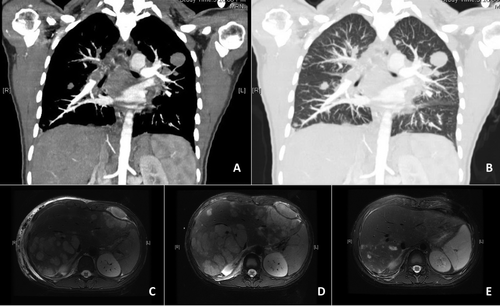
At age 19, the patient presented with fever, cough, and chest pain. Her chest X-ray showed bilateral pulmonary nodules. A computed tomography (CT) of her chest revealed bilateral pulmonary and pleural nodules with extensive mediastinal and hilar lymphadenopathy, as well as a heterogeneously enhancing liver (Fig. 1A,B). Her liver function tests (LFTs) were slightly elevated as well. She was admitted, and an extensive infection workup was negative for mycobacteria, mycoplasma, Legionella, Aspergillus, Cryptococcus, Pneumocystis, respiratory viruses, hepatitis B, hepatitis C, human immunodeficiency virus, and cytomegalovirus. Quantitative PCR for the EBV gene Epstein-Barr nuclear antigen (EBNA)-1 indicated that viral levels were elevated (2900 IU/mL). She was started on valganciclovir, and her tacrolimus was discontinued.
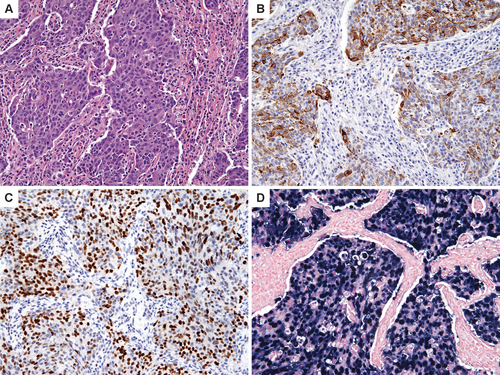
She underwent a thoracoscopic right lung biopsy. Several nodules up to 2 cm were seen on both the lung parenchyma and the parietal pleura. The pleuritic fluid was negative for all infectious agents. The biopsy specimen showed a poorly differentiated carcinoma characterized by nests of epithelial cells admixed with dense reactive lymphoid infiltrates (Fig. 2A). Extensive immunohistochemical analysis revealed positive staining for cytokeratin 5/6 and p63, confirming the epithelial differentiation and suggesting a “lymphoepithelioma-like” carcinoma, similar to that usually observed in the nasopharynx (Fig. 2B,C). There was no immunohistochemical support for primary lung carcinoma. In addition, the carcinoma was EBV-positive by Epstein-Barr virus-encoded small RNAs (EBERs) in situ hybridization (Fig. 2D). The specimen was also sent out to the Rare Tumor Board at the National Institutes of Health (NIH), who agreed with the diagnosis of LEC.
The patient was discharged but readmitted within 2 weeks with high fevers, worsening cough, and tachycardia to 150 beats per minute. Her EBV level was now 29,000 IU/mL. She was found to have a new malignant pleural effusion, which was drained. A biopsy of her liver was negative for graft rejection but demonstrated LEC, with histology similar to her lung lesions. Magnetic resonance imaging (MRI) showed marked progression of the carcinoma in her liver since initial presentation (Fig. 1C,D).
The patient was started on induction chemotherapy with cisplatin and fluorouracil, based on a protocol for the histologically similar nasopharyngeal carcinoma. After just 1 cycle, a repeat MRI showed radiological improvement, with a reduction in the number and size of metastatic lesions throughout the liver (Fig. 1D,E). Her LFTs had also normalized at this time. After her second cycle of chemotherapy, however, the patient decided not to complete the chemotherapy regimen because of the side effects of the medications in the setting of a dismal prognosis. She received end-of-life care and shortly died from complications of her malignancy.
Results
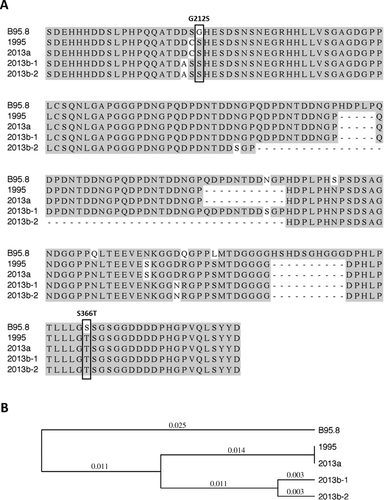
The availability of patient blood samples from both 15 months and 19 years of age allowed sequencing of her EBV genome to track specific viral species. When we sequenced the EBV-encoded proto-oncogene latent membrane protein 1 (LMP1) from the sample obtained at age 19, during the workup of her de novo carcinoma, we found 3 closely related LMP1 sequences, suggesting concurrent infection with 3 related viral species (Fig. 3). Interestingly, 1 of these variants contained LMP1 sequences that were identical to LMP1 sequences isolated at the time of her initial EBV infection at 15 months of age, suggesting that the original viral species persisted. The 2 other LMP1 sequences appear to represent viral derivatives of the original infection that developed in the intervening 18 years.
Discussion
Although posttransplant patients can develop a variety of de novo malignancies, this is the first reported case of posttransplant LEC, to our knowledge. LEC is composed of syncytial sheets of large, pleomorphic, and undifferentiated epithelial cells with a rich stromal infiltrate of inflammatory cells. These infiltrating cells are mostly lymphocytes and plasma cells, which are reactive and polyclonal in nature and, hence, are not themselves neoplastic.
This was a surprising diagnosis. It is unknown why our patient developed LEC after 18 years of relatively good health, even though her Asian ethnicity, EBV positivity, and chronic immunosuppression were certainly risk factors. The more common EBV-associated posttransplant malignancy, PTLD, by comparison, usually develops within 1 to 2 years of transplantation. In addition, the mean age of newly diagnosed LEC patients is the fifth decade of life, whereas our patient was only a teenager. Lastly, it is unknown whether our patient's lung or liver was the primary site of the carcinoma or whether they were synchronous tumors. Either location would be quite rare for LEC, which is usually found in the salivary glands or the larynx. In fact, to date, there have been approximately only 150 reported patients with primary LEC in the lung,2 and none in the liver parenchyma.
On a population level, the association between LEC and EBV is actually somewhat controversial. In general, it appears that LEC in patients of Chinese, Southeast Asian, and Eskimo ethnicities are strongly associated with EBV, whereas the association is much weaker for patients of European or Caucasian descent.3 The site of the carcinoma is also important because LECs of the salivary gland and lung are more commonly associated with EBV than LECs originating from other sites. Therefore, in our patient, both her ethnicity and her tumor site would favor the link between her LEC and EBV, an association confirmed by histology.
LMP1 is an oncoprotein that is crucial to the transformation of B lymphocytes and is well studied in the setting of PTLD. Less is known about the role of LMP1 in LEC. Jen et al.4 found several specific mutations, including a glycine to serine change at position 212 (G212S) and a serine to threonine change at position 366 (S366T) in LMP1 that are conserved across both salivary gland LEC LMP1 and its histologically similar nasopharyngeal carcinoma LMP1, which distinguish both strains of EBV from non-oncogenic EBV strains. These 2 specific point mutations were also present in the LMP1 sequences isolated from our patient at both time points (Fig. 3A). In addition, our laboratory has previously identified these 2 mutations in EBV genomes isolated from PTLD patients, and we showed that these are gain-of-function mutations because they are linked to sustained ERK/MAP kinase activation and induction of the c-fos oncoprotein in B cells.5
Treating these de novo malignancies remains a challenge. Reducing or withdrawing immunosuppression, which is an accepted first-line intervention for posttransplant cancers, was ineffective in our patient, as her tumor continued to grow (Fig. 1C,D). The use of antiviral therapy for virus-related malignancies is controversial. In the present patient, valganciclovir was administered to treat EBV viremia in a sick patient at a time when the underlying cause of her symptoms was still unknown; the cancer had not been found yet. Once the diagnosis was confirmed, and after consultation with the NIH, the decision was made to start the patient on chemotherapy.
Although the modalities used to treat these posttransplant malignancies are similar to those used to treat the general population, there are unfortunately additional adverse effects for transplant recipients. Chemotherapy may further immunosuppress patients and increase their risk for infections. Some regimens may even be harmful to the allograft. Lastly, these patients have already experienced the triple burden and anxiety of organ failure, a major transplant operation, and secondary malignancy, and therefore may be faced with fatigue and disillusionment with further treatments and their side effects, as was the case with our patient.
This patient reminds us that chronic immunosuppression in an EBV-positive patient can result in the development of a variety of EBV-related malignancies, including PTLD and other rare cancers, even after decades of EBV inactivity. We use this patient to highlight LEC as a rare carcinoma that clinicians who are working up their transplant patients for possible malignancy can consider on their differential. As the life expectancy of pediatric liver recipients increases and as our current recipient population ages, we must develop evidence-based guidelines on how to best screen for de novo malignancies in these patients.