Successful hepatocellular carcinoma downstaging with transarterial chemoembolization followed by stereotactic radiotherapy
Potential conflict of interest: Nothing to report.
Abbreviations
-
- CPS
-
- Child-Pugh score
-
- CT
-
- computed tomography
-
- Gy
-
- gray
-
- HCC
-
- hepatocellular carcinoma
-
- HE
-
- hepatic encephalopathy
-
- LFT
-
- liver function test
-
- MC
-
- medically controlled
-
- MELD
-
- Model for End-Stage Liver Disease
-
- mRECIST
-
- modified response evaluation criteria in solid tumors
-
- PE
-
- pleural effusion
-
- POD
-
- progression of disease
-
- SRT
-
- stereotactic radiotherapy
-
- TACE
-
- transarterial chemoembolization
Approximately 16%-18% of liver transplants in the United States were performed for the oncologic treatment of hepatocellular carcinoma (HCC).1 Although transarterial chemoembolization (TACE) was the most common HCC bridging locoregional therapy while on the wait list (75%), TACE rarely sterilizes HCC, especially in larger tumors.1 Studies suggest that only 24%-90% of patients were successfully downstaged from stage T3-4 tumors to within Milan criteria using TACE as a sole downstaging modality.2 It is now appreciated that the usage of more than 1 locoregional therapy may be most effective in successful HCC downstaging of beyond Milan criteria tumors before liver transplantation.
Radiation therapy is increasingly being used to treat large liver tumors where modern computer technology has overcome some of the historic drawbacks via accurate and targeted dose-delivery.3 The stereotactic radiotherapy (SRT) technique delivers treatment typically over 3-5 treatments, compared to the standard duration of 20-25 treatments over a 4– to 5–week period. Accurate delivery of high doses to a small target over fewer treatments has been demonstrated to result in improved liver tumor control and reduced toxicities.3 Although TACE and SRT can be used independently, a combination of these therapies maximizes the strengths and reduces the limitations of these individual treatments.4
We previously reserved the combination of TACE and SRT to large inoperable HCC patients who were not eligible for liver transplantation.4 After being impressed with the effectiveness of this treatment combination, we began to use this approach for downstaging beyond Milan criteria HCC patients in an attempt to make them eligible for liver transplantation. The purpose of this study is to report a case series of 12 HCC patients who were treated with TACE followed by SRT in attempt to successfully down-stage for liver transplantation.
Methods
Treatment Approach
An a priori decision to use TACE followed by SRT to down-stage HCC patients so they could be considered for liver transplantation was made at a multidisciplinary tumor board comprising of transplant surgeons, hepatologists, radiologists, interventional radiologists, nuclear medicine physicians, medical oncologists, and radiation oncologists.
When SRT was planned to follow TACE, conventional TACE with lipiodol and chemotherapy was used. Lipiodol is visible on fluoroscopy and noncontrast computed tomography (CT), which facilitates localization of the target for image-guided radiotherapy. Targeted super-selective TACE was performed initially in all patients. Briefly, a microcatheter was advanced to the tumor-feeding artery or arteries. Nontumor liver tissue was spared as much as possible. A mixture of 50 mg of doxorubicin and lipiodol was delivered until the tumor was saturated. This is followed with 400-μm Embozene microsphere particles (CeloNova BioSciences, San Antonio, TX) to achieve complete stasis. The lipiodol used during embolization typically remains in the tumor for several months when administered as described above (Fig. 1).
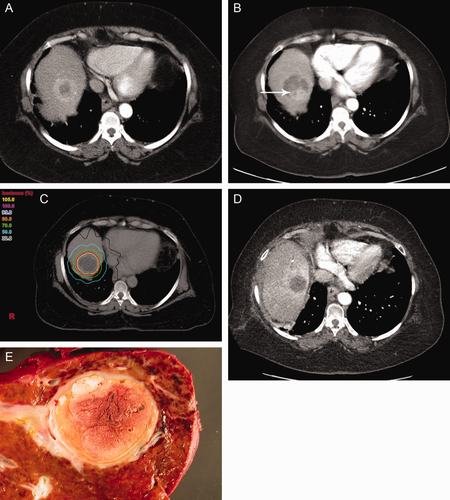
Example of wait-listed patient with a beyond Milan criteria HCC treated with TACE followed by SRT. (A) Initial CT imaging of HCC tumor, (B) CT imaging of HCC tumor after TACE treatment demonstrating Modified Response Evaluation Criteria in Solid Tumorsv (mRECIST) partial tumor necrosis with arrow denoting persistent viable tumor, (C) CT imaging illustrating SRT planning, (D) CT imaging of HCC tumor post-SRT treatment demonstrating mRECIST complete tumor necrosis, and (E) gross explant pathology of the HCC tumor after treatment with TACE followed by SRT therapy with no viable tumor detected microscopically.
If the tumor-lipiodol was not well visualized following TACE, patients underwent CT-guided placement of liver fiducials (Civco Polymark 1 mm × 3 mm fiducials; Civco Medical Solutions; Orange City, IA) to facilitate image registration and tumor targeting at the time of radiation treatment.
SRT for HCC was typically performed using a 3-fraction schedule for a total of 45 Gray (Gy), delivered over 10 calendar days. Clinically accepted practices were followed including custom immobilization, treatment planning, respiratory correction, and image-guided treatment delivery.4 Fixed-field intensity-modulated radiation therapy or dynamic arc plans were used for treatment delivery on a linear accelerator. At least 800 cc of normal liver parenchyma volume was spared to receive less than 15 Gy during SRT. Changes in SRT dosing were made only when 800 cc of uninvolved liver could not be spared, or dose-tolerance limits to adjacent critical organs could not be met.
Case Series
There were 12 patients who were treated with TACE-SRT combination, with a mean follow-up of 29 months (range 6-48; Table 1). These patients (9 male, 3 female) were not initially transplant candidates because their tumors exceeded the Milan criteria. The mean age of the patient group was 58.6 years (range, 42-70 years). The Model for End-Stage Liver Disease (MELD) score ranged from 6 to 14, and no patients had clinically evident hepatic decompensation symptoms. HCC diagnosis was made based on Organ Procurement and Transplant Network criteria: one 5a tumor (6 patients), two 5b tumors (5 patients), one 5b and one 5a tumors (1 patient). In addition to meeting imaging criteria, 4 of the patients had an alpha-fetoprotein level over 100 and the 4 patients had biopsy-proven HCC. The underlying liver disease was hepatitis B (n = 3), hepatitis C (n = 6), alcohol (n = 1), alpha-1-antitrypsin (n = 1), and nonalcoholic steatohepatitis (n = 1). A total of 18 tumors were treated in these 12 patients, sizes ranging from 1.7 to 7.6 cm (mean, 4.2 cm). A single TACE procedure was performed in 7 patients, whereas the remaining 5 patients had 2 TACE procedures performed to completely embolize the HCC before SRT. Though thermal ablation was typically used at our institution to treat HCC < 3 cm, 5 patients in this series had lesions < 3 cm that were too close to major blood vessels or the biliary tree, and hence were treated with SRT. The mean time from diagnosis to first TACE was 7.8 weeks (range, 3-24) and from TACE to SRT was 13 weeks (range, 6-18). The mean biological equivalent dose of radiation delivered using SRT was 255 Gy3 (range, 180-270 Gy3). The mean time from SRT to liver transplantation was 7.8 months.
| Patient | Age and Sex | MELD/CPS | Baseline Decompensation | Tumor Size, cm | Segment Location | Decompensation After TACE-SRT | TACE Procedures | SRT Localization | SRT Doses | Transplant Status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 63-year-old male |
MELD 12 CPS-C |
MC-HE, varices | 3.6; 2.2 | 5; 8 | MC-HE, varices | 2 | Lipiodol | 15 Gy, 3 doses | Transplanted |
| 2 | 60-year-old female |
MELD 7 CPS-A |
None | 3.9; 2.2 | 6; 5 | MC-HE, MC ascites | 2 | Lipiodol | 15 Gy, 3 doses | Transplanted |
| 3 | 42-year-old male |
MELD 6 CPS-A |
None | 4.5; 3.0 | 6/7; 5 | None | 1 | Lipiodol | 15 Gy, 3 doses | Transplanted |
| 4 | 70-year-old male |
MELD 6 CPS-A |
None | 6.0 | 8 | None | 1 | Lipiodol | 15 Gy, 2 doses | Transplanted |
| 5 | 53-year-old female |
MELD 8 CPS-B |
Varices | 4.7; 1.7 | 7; 8 | Varices | 2 | Fiducials | 15 Gy, 3 doses | Transplanted |
| 6 | 66-year-old male |
MELD 7 CPS-A |
None | 5.5 | 4/5/8 | None | 1 | Lipiodol | 15 Gy, 3 doses | Transplanted |
| 7 | 57-year-old male |
MELD 6 CPS-A |
None | 4.7 | 5/8 | None | 1 | Fiducials | 15 Gy, 3 doses | Active on wait list |
| 8 | 58-year-old male |
MELD 9 CPS-B |
Varices | 5.7 | 5/6 | MC-HE, MC-PE, renal failure | 1 | Lipiodol | 15 Gy, 3 doses | Died of sepsis 2 months after SRT |
| 9 | 60-year-old female |
MELD 13 CPS-B |
None | 7.6 | 7 | None | 2 | Lipiodol | 15 Gy, 3 doses | POD outside of SRT field |
| 10 | 61-year-old male |
MELD 9 CPS-B |
PE | 4.5; 2.0 | Central right lobe 5/6/7/8 | MC ascites | 1 | Fiducials | 13.5 Gy, 2 dosesa | POD outside of SRT field |
| 11 | 55-year-old male |
MELD 14 CPS-C |
Jaundiced | 6.0 | 5/6/7 | Ascites | 1 | Lipiodol | 7.5 Gy, 6 dosesb | POD outside of SRT field |
| 12 | 58-year-old male |
MELD 10 CPS-A |
None | 5.2; 2.4 | 5/8 | Varices, ascites | 2 | Lipiodol | 15 Gy, 3 doses | POD at edge of SRT field |
- a Chosen due to a smaller than average total liver volume.
- b Chosen due to concern for acute toxicity in the setting of poor LFTs.
Six patients have undergone liver transplantation. The liver transplant operations were straightforward with 3/6 recipients receiving no blood products (mean red blood cell transfusion, 3.2 units). There were no complications with the hepatic artery reconstruction. Two of the 6 patients developed biliary strictures after transplant, which were managed endoscopically. One patient developed acute cellular rejection within 90 days of transplantation managed with pulse steroid therapy. Explant pathology demonstrated pathological complete response, with no viable tumor detected in the 10 treated HCC sites in the 6 case series patients (100% necrosis). However, there was viable HCC tumor present in 2 patients in areas of the liver that were not treated with either TACE or SRT. In 1 patient, there was evidence of small diameter (<1 cm) multifocal HCC involving both lobes, whereas in another patient, there was a single focus of additional HCC (1.4 cm) away from the treated site, not apparent on the diagnostic imaging. One patient died without evidence of tumor recurrence at 29 weeks following liver transplantation due to medication noncompliance. Five patients are alive and without signs of tumor recurrence at the time of reporting.
Six patients did not undergo transplantation (Table 1). One patient is active on the transplant wait list. One patient died of sepsis 2 months after SRT. Three patients developed new HCC tumors outside the SRT treatment field while on the wait list and were delisted. In each of these 3 patients, the TACE+SRT-treated HCC tumors demonstrated a complete radiographic response based on mRECIST criteria. One patient developed progressive tumor growth at the margin of the SRT treatment field and also was delisted.
Discussion
This limited case series demonstrates the effectiveness of TACE followed by SRT in downstaging beyond Milan criteria HCC. TACE followed by SRT was completed in all 12 patients. Six patients underwent liver transplantation after downstaging, and perhaps most compelling, no viable tumor was detected on explant pathology in the treated HCCs. Furthermore, the SRT-treated tumors demonstrated a complete radiographic response in the 3/4 patients delisted (for tumor progression elsewhere in the liver). Only 1 patient demonstrated tumor progression at the margin of the SRT treatment field. Although TACE and SRT can be used independently, a combination of these therapies is complimentary and has a number of potential advantages. TACE-related treatment failures are expected to occur at the periphery of the treated tumor, which often has supplemental vascular supply, whereas SRT-related treatment failures typically occur at the center, where the tumor is most hypoxic.4 Initial TACE treatment typically results in tumor involution and can help reduce radiation fields thus sparing uninvolved hepatic parenchyma. In addition, cytotoxic agents used in TACE can exert a local radio-sensitization effect, and TACE-SRT combination may be synergistic.
Perhaps the pragmatic clinical debate is whether or not it is necessary to use SRT as HCC downstaging adjunct to TACE. There are no randomized clinical trials to inform clinical decision-making. In this limited case series, only 1 patient died following TACE+SRT. Low complication rates have been reported among studies of SRT5, 6; thus, the treatment regimen appears safe even in the setting of cirrhosis and liver decompensation. A transplant center's approach is based on their philosophy as to whether it is acceptable to simply shrink a HCC tumor while on the wait list or aim for complete tumor necrosis. The tumor response rate following TACE+SRT was significantly higher than similar studies using SRT alone as a bridge to transplant.5, 6 Furthermore, the nontransplant HCC literature demonstrates a survival advantage to treating the periphery of TACE-treated HCC tumors with an additional locoregional therapy. For small HCC tumors, ablation has been demonstrated to result in increased HCC necrosis and improved survival. However, technical failure with HCC tumors ≥3 cm or centrally located lesions are common. Our center uses SRT following TACE treatment of HCC tumors >3.0 cm.4 SRT dosage may need to be modified for peripheral tumors adjacent to the heart or hollow viscus. A recent meta-analysis which studied 17 clinical trials treating over 1476 patients using either TACE or a combination of TACE with conventional radiation therapy demonstrated a significant improvement in overall survival with the use of the combined therapy compared to TACE alone.7 These studies demonstrate improved survival in nontransplant patients although the role of SRT as a bridge to transplant remains unclear.
In conclusion, this limited case series suggests that TACE followed by SRT may be an effective downstaging approach for select patients with HCC tumors >3 cm that exceed the Milan criteria HCC.
Acknowledgements
This research was funded by National Institutes of Health grant numbers 1 K23 DK091514 (DD) and 1 R03 DK106432 (DD).
-
Rojymon Jacob, M.D.1
-
Souheil Saddekni, M.D.2
-
Laura Dover, M.D.1
-
Derek A. DuBay, M.D.3
-
Departments of 1Radiation Oncology,
-
2Radiology-Interventional Oncology, and
-
3Surgery-Transplantation
-
University of Alabama Birmingham
-
Birmingham, AL