Liver transplantation in the mouse: Insights into liver immunobiology, tissue injury, and allograft tolerance
Grants and financial support: The authors' work was supported by National Institutes of Health (NIH) grants P01 AI81678 and R56 AI126377. Osamu Yoshida was supported by NIH institutional training grant T32 AI74490.
Potential conflict of interest: Nothing to report.
Abstract
The surgically demanding mouse orthotopic liver transplant model was first described in 1991. It has proved to be a powerful research tool for the investigation of liver biology, tissue injury, the regulation of alloimmunity and tolerance induction, and the pathogenesis of specific liver diseases. Liver transplantation in mice has unique advantages over transplantation of the liver in larger species, such as the rat or pig, because the mouse genome is well characterized and there is much greater availability of both genetically modified animals and research reagents. Liver transplant experiments using various transgenic or gene knockout mice have provided valuable mechanistic insights into the immunobiology and pathobiology of the liver and the regulation of graft rejection and tolerance over the past 25 years. The molecular pathways identified in the regulation of tissue injury and promotion of liver transplant tolerance provide new potential targets for therapeutic intervention to control adverse inflammatory responses/immune-mediated events in the hepatic environment and systemically. In conclusion, orthotopic liver transplantation in the mouse is a valuable model for gaining improved insights into liver biology, immunopathology, and allograft tolerance that may result in therapeutic innovation in the liver and in the treatment of other diseases.
Abbreviations
-
- Ag
-
- antigen
-
- APC
-
- antigen-presenting cell
-
- AR
-
- amphiregulin
-
- ATP
-
- adenosine triphosphate
-
- B7-H1
-
- B7 homolog 1
-
- BM
-
- bone marrow
-
- BW
-
- body weight
-
- CCL
-
- chemokine (C-C motif) ligand
-
- CTL
-
- cytotoxic T lymphocyte
-
- DAP12
-
- DNAX-activating protein of 12 kDa
-
- DAPI
-
- 4',6-diamidino-2-phenylindole
-
- DC
-
- dendritic cell
-
- DR
-
- death receptor
-
- DT
-
- diptheria toxoid
-
- DTR
-
- diptheria toxoid receptor
-
- FasL
-
- fas ligand
-
- FH
-
- fulminant hepatitis
-
- Flt3L
-
- fms-like tyrosine kinase 3 ligand
-
- Gal-9
-
- galactin-9
-
- GFP
-
- green fluorescent protein
-
- HH
-
- hereditary hemochromatosis
-
- HO-1
-
- hemoxygenase-1
-
- IFNγ
-
- interferon γ
-
- IFNAR
-
- type 1 interferon receptor
-
- IL
-
- interleukin
-
- I/R
-
- ischemia/reperfusion
-
- IRF-1
-
- interferon regulatory factor-1
-
- IRI
-
- ischemia/reperfusion injury
-
- JNK
-
- c-Jun N-terminal kinase
-
- KC
-
- Kupffer cell
-
- KO
-
- knockout
-
- LN
-
- lymph node
-
- lpr
-
- lymphoproliferation
-
- mDC
-
- myeloid dendritic cell
-
- MHC
-
- major histocompatibility complex
-
- mRNA
-
- messenger RNA
-
- NIH
-
- National Institutes of Health
-
- NK
-
- natural killer
-
- NKT
-
- natural killer T cell
-
- OT-1
-
- ovalbumin-specific CD8+ T cells
-
- PBS
-
- phosphate-buffered saline
-
- PD-L1
-
- programmed death–ligand 1
-
- SIINFEKL
-
- ovalbumin-derived 8-residue peptide
-
- SFSS
-
- small-for-size syndrome
-
- TCA-3
-
- T cell activation gene-3
-
- tg
-
- transgenic
-
- TIM
-
- T cell immunoglobulin and mucin
-
- TLR
-
- toll-like receptor
-
- TNF-α
-
- tumor necrosis factor α
-
- Treg
-
- regulatory T cell
-
- WT
-
- wild-type
Mouse Liver Transplant Model and its Significance
Since it was first described by Qian et al.1 at the University of Pittsburgh in 1991, mouse liver transplantation, a challenging surgical model, has provided a pathway to find key mechanistic insights into liver immunobiology, regulation of hepatic injury, and the balance between organ transplant rejection and tolerance. A survey of the literature performed in October 2015 revealed at least 70 articles describing the model to study not only the mechanisms underlying liver transplant ischemia/reperfusion (I/R) injury and alloimmunity/“spontaneous” tolerance, but also other aspects of liver biology, immunology, and pathology. These include liver regeneration, hepatic immune cell homeostasis, antigen (Ag) presentation, and the pathogenesis of certain liver diseases (fulminant hepatitis [FH] and hemochromatosis). Here, we provide a concise review of the significant insights gained using the model over the past 25 years.
Alloimmunity and Mechanisms Underlying Liver Allograft Tolerance
The liver is a unique anatomical and immunological site.2, 3 In mice, liver allografts are accepted indefinitely across major histocompatibility complex (MHC) barriers without immunosuppressive therapy.4 “Operational tolerance” also occurs in the clinic in approximately 20% of stable human liver allograft recipients intentionally weaned off all immunosuppressive agents.5-7 This tolerogenic effect is sufficiently robust and specific in mice that subsequent skin or heart allografts from the same donor strain are also accepted, whereas third-party grafts are rejected.4, 8 In addition, liver transplantation can prevent accelerated rejection after repeated skin transplantation in sensitized recipients and protect such “second-set” skin grafts permanently.9 Although other types of organ allografts (ie, kidneys or hearts) may also induce tolerance in mice, liver allografts exert a much stronger and reproducible effect.9, 10 Much effort has been made to delineate the cellular and molecular basis of allograft tolerance, with a particular focus on the roles of donor-derived or host immune cells. Thus, for example, the Fas/FasL system appears to play an important role in host immune regulation, enabling liver allograft acceptance by deletion of donor-specific cytotoxic T lymphocytes (CTLs).11 The cells and molecular mechanisms that have been implicated in regulating the balance between tolerance and rejection in the mouse liver transplant model are summarized in Table 1 and depicted in Fig. 1.
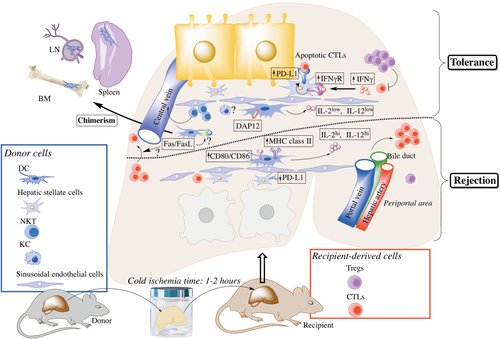
Cellular and molecular mechanisms implicated in regulating the balance between tolerance and rejection in the mouse liver transplant model. Donor-derived cells, such as DCs, are found in lymphoid tissues in long-surviving, fully allogeneic mouse liver transplant recipients. Donor-derived DC and NKTs, but not KCs, have been reported to play an important role in the establishment of mouse liver transplant tolerance. Enhanced production of IL12 by donor DC (as the result of Flt3L administration to the liver donor) or exogenous IL2 can promote CTLs and leads to graft rejection. The transmembrane adaptor protein DAP12 expression by the allograft appears to be a critical molecule that regulates the induction of liver transplant tolerance because its absence results in enhanced host CTLs and reduced Tregs. Similarly, IFNγ signaling appears to be required for mouse liver allograft acceptance; host-derived IFNγ induces PD-L1 up-regulation on donor-derived stellate cells that, in turn, promote apoptosis of graft-infiltrating CTLs. The Fas/FasL pathway also appears to play a critical role in mouse liver transplant tolerance, but the exact mechanisms have not yet been elucidated.
| Cells or Molecules | Findings Regarding Transplant Tolerance | Reference |
|---|---|---|
| Donor-derived cells | ||
| DCs | Systemic donor-derived hematopoietic cell chimerism in liver transplant host lymphoid tissues | Qian et al.9 (1994) |
| Propagation of donor DC from lymphoid tissue of liver transplant but not heart transplant recipients | Lu et al.12 (1995) | |
| Proposed immunological role of donor-derived DC in BM chimerism in host | Thomson et al.13 (1995) | |
| Marked increases in DC within the graft induces rejection that can be reversed with IL12 blockade | Li et al.14 (2001) | |
| DAP12 expression by liver DCs may be critical for the induction of tolerance | Yoshida et al.15 (2014) | |
| KCs | KCs are not critical for tolerance | Morita et al.16 (2015) |
| NKTs | NKTs play a key immunomodulatory role | Morita et al.17 (2007) |
| Donor NK cells and NKTs remain in liver and blood and are resistant to rejection | Tay et al.18 (2013) | |
| Hepatic stellate cells | Up-regulated PD-L1 expression by stellate cells is important in tolerance induction | Morita et al.16 (2015) |
| Host cells | ||
| CTLs | Apoptosis of CTLs within the grafts may provide a mechanistic basis of allograft acceptance | Qian et al.19 (1997) |
| Tregs | Depletion of host Tregs increases alloimmune responses | Jiang et al.20 (2006) Li et al.21 (2008) |
- NOTE: In addition, expression of Fas (CD95) and FasL by various immune cells (and hepatocytes) may play an important role in regulation of antidonor CTL responses.11
Role of Donor-Derived Hematopoietic Cells in Mouse Liver Transplantation Tolerance
Although whole liver allografts have well-established tolerogenic properties, purified mouse hepatocytes are immunogenic and elicit rejection,22, 23 suggesting that nonparenchymal cells play a key role in liver transplant tolerance. These donor-derived hematopoietic cells migrate rapidly from the graft following transplantation and are found in the blood as early as 1.5 hours after transplant and in the spleen and lymph nodes (LNs) 1.5 to 24 hours after transplant.24 Interestingly, distinct leukocyte populations exhibit different migration patterns. Thus, donor T and B cells migrate to the LNs and spleen, whereas natural killer (NK) cells and natural killer T cells (NKTs) remain in the liver and blood. Reductions in donor T cells in both LNs and spleen suggest that these lymphocytes may be rejected rapidly, whereas persistence of NK cells and NKT in the blood and the liver suggests that these cells are resistant to rejection.18
An important role of donor-derived hematopoietic cells was further suggested by the observation that long-surviving, fully allogeneic mouse liver transplant recipients exhibit systemic hematopoietic cell chimerism (ie, the presence of donor-derived leukocytes in host tissues) several months after transplant.9 Donor-derived cells are found most readily in host lymphoid tissues (eg, the spleen, mesenteric LNs, and thymus).12 Small numbers are also found in the small bowel, skin, kidney, tongue, heart, and lung.9 Donor cells can also be detected, although the frequency of cells expressing donor MHC class I is very low (1%), in host bone marrow (BM) 2 weeks after mouse liver transplantation in the absence of immunosuppressive therapy.12, 13 These donor-derived populations include not only innate immune cells (dendritic cells [DCs] and macrophages), but also T and B lymphocytes that may also have immunoregulatory properties.9
Significantly, donor-derived professional Ag-presenting cells (DC) can be propagated from lymphoid tissue of mouse liver allograft recipients but not from mice that acutely reject cardiac allografts from the same donor strain in the absence of immunosuppression.12 This suggests that liver tolerogenicity may depend on donor-derived hematopoietic cells (in particular DCs25 that can inhibit the host's adaptive immunity).12, 24, 26, 27 Confirmation of the crucial importance of donor liver DCs in the promotion of mouse liver transplant tolerance comes from experiments in which conventional CD11c+ DCs have been depleted selectively from liver allografts before their transplantation. As shown in Fig. 2, livers from DC-depleted donors are rejected acutely, whereas those from wild-type (WT) donors are accepted indefinitely. On the other hand, when livers with dramatically increased numbers of interstitial DCs (the result of Fms-like tyrosine kinase 3 ligand administration) are transplanted into WT recipients, they are also rejected acutely.14, 28 However, in contrast to normal livers, these allografts exhibit marked up-regulation of interleukin (IL) 12 production by interstitial donor DCs.14 When IL12 is neutralized, graft survival is markedly prolonged, which is associated with the inhibition of intragraft donor-specific CTL and NK cell activities and with CD8+ T cell apoptosis in the graft and lymphoid tissue. These results indicate that, in this experimental setting, marked increases in numbers of migratory liver-derived DCs that exhibit enhanced IL12 production can switch tolerance to rejection.14
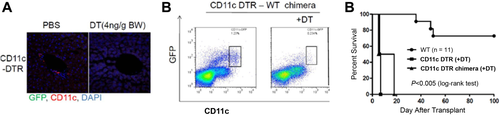
Profound depletion of interstitial (periportal) CD11c+ conventional mDC following DT administration in (A) the liver of a B6 CD11c-DTR mouse and (B) the liver of a chimeric B6 mouse in which only the hematopoietic cells were CD11c-DTR. (C) Survival of liver allografts from mDC-depleted B6 CD11c-DTR donor mice or from mDC-depleted B6 donor mice (CD11c-DTR chimeras) in which only the hematopoietic cells were CD11c+-DTR. Recipients were WT C3H mice. Experiments were conducted under an Institutional Animal Care and Use Committee–approved protocol (no. 130590681).
Several molecular mechanisms, whereby donor hematopoietic and other liver cells may promote transplant tolerance, have been identified. Recently, the transmembrane adaptor protein DNAX-activating protein of 12 kDa (DAP12) has been shown to regulate the maturation of liver DC, their ability to migrate to host lymphoid tissue, and their T cell allostimulatory capacity.15, 29 Thus, freshly isolated DAP12-/- liver myeloid dendritic cells (mDCs) exhibit a more mature phenotype, enhanced secretion of proinflammatory cytokines, and greater naïve T cell allostimulatory activity than WT liver DCs. Following liver transplantation from DAP12-/- (B6; H2b) donors to WT C3H (H2k) recipients, donor liver mDCs migrate in greater numbers to host spleens, which is associated with increased proinflammatory cytokine (IL6, IL12p40, perforin, granzyme B, tumor necrosis factor [TNF]–α, and interferon [IFN] γ) expression in the grafts. Moreover, DAP12-/- liver grafts fail to induce tolerance and are rejected acutely.15 These results suggest that DAP12 expression in donor livers is important in regulating donor DC migration to host lymphoid tissue, modulation of alloreactive T cell responses, and the induction of tolerance.
The role of other liver-resident antigen-presenting cells (APCs) in the induction of transplant tolerance has also been investigated. Thus, the function of hepatic macrophages (Kupffer cells [KCs]) has been examined by selectively depleting these cells in donor mouse livers by clodronate liposome administration. However, these KC-depleted liver allografts are still accepted spontaneously,16 indicating that donor KCs are not critical for induction of liver transplant tolerance in mice.
Donor-derived NKTs may play an important role in the establishment of mouse liver transplant tolerance. Thus, when liver grafts from NKT (Jα281) knockout (KO) mice on the C57BL/6 (B6; H2kb) background are transplanted into WT BALB/c (H2kd) mice (NKT KO→WT), only 50% of the recipients survive, whereas 91% of WT B6 donor grafts survive in WT BALB/c (WT→WT) recipients. WT recipients of NKT KO livers also exhibit extensive lymphocytic infiltration of portal triads and bile duct epithelium, which is largely absent in the WT→WT combination.17 These results indicate that donor-derived NKT, like DC, may play a key immunomodulatory role in the development of mouse liver allograft tolerance.
A recent report16 shows that IFNγ signaling, reported earlier to be an absolute requirement for mouse liver allograft acceptance,30 up-regulates coinhibitory B7 homolog 1 (B7-H1 Programmed death–ligand 1 [PD-L1]) expression on donor-derived CD45- hepatic stellate cells and that apoptosis of graft-infiltrating CD8+ T cells is important in tolerance induction. The findings indicate that T effector cell-derived IFNγ may be critical in initiating a negative feedback loop via PD-L1 that, in turn, promotes apoptotic elimination of T effectors and tolerance induction.16 These results are in accord with previous studies from the same group in which PD-L1-/- liver transplantation resulted in rejection.31
Role of Host Immune Cells in Mouse Liver Transplantation Tolerance
Most donor passenger leukocytes exit mouse liver grafts and are rapidly replaced by recipient leukocytes within 1.5 hours of transplantation.18 Along with an initial, nonspecific increase in myeloid cells (predominantly neutrophils and granulocyte receptor 1+ monocytes), liver allografts exhibit increased numbers of T and B cells.18 By day 4 after transplant, 95% of T cells (mainly CD4+) are recipient-derived.19 At later time points (days 7 and 14), CD8+T cells predominate. Alloreactive CTLs observed on day 4 after transplant decline gradually over time19 associated with increases in apoptotic graft-infiltrating T cells that appear to contribute to tolerance induction. Thus, systemic administration of mouse IL2 that reduces apoptotic cells within the graft-infiltrating cell population, increases CTL activity, and induces acute graft rejection.19, 32
The role of host regulatory T cells (Tregs) in mouse liver allograft acceptance has also been studied.20, 21 Thus, CD4+CD25+ forkhead box P3+ T cells are increased in the grafts and host spleens compared to naive mice between days 5 and 100 after transplant.21 They appear to be important in the induction of tolerance because their selective depletion (but not depletion of donor Treg) abrogates tolerance.20, 21 Moreover, depletion of host CD4+CD25+ T cells increases CD4+ and CD8+T cell responses with reduced apoptosis of graft-infiltrating T cells.20, 21
Liver Transplant Ischemia/Reperfusion Injury
Graft ischemia/reperfusion injury (IRI) remains as an important problem during liver transplantation.33, 34 Que et al.35 developed a mouse model to study cold IRI in the liver by performing orthotopic liver transplantation with various cold preservation times. Thirty-day graft survival was 100%, 100%, 88%, and 0% for 1, 4, 8, 16 hours preservation, respectively. Prolonged cold preservation induced more IL6 expression and bromodeoxyuridine uptake in the grafts and resulted in severe liver injury. Shen et al.36 found that hepatic artery reconstruction reduced cold IRI and resulted in less CD4+ T cell infiltration, with reduced cytokine expression and hepatocellular damage.
Xie et al.37 have reported that partial liver grafts display more severe cold IRI in the liver compared with whole grafts. Although expression of chemokines, including T cell activation gene-3 (TCA-3, chemokine [C-C motif] ligand [CCL] 1), is higher in partial grafts, neutralization of TCA-3 decreases neutrophil and T cell infiltration and attenuates cold IRI.
Molecular Regulation of Ischemia/Reperfusion Injury
Molecular mechanisms that have been implicated in liver IRI on the basis of studies in the mouse liver transplantation model are summarized in Table 2 and depicted in Fig. 3. Interferon regulatory factor-1 (IRF-1), a ubiquitous nuclear transcription factor induced by several agents (cytokines, hormones, viruses, and retinoic acid), regulates cell proliferation, apoptosis, and immune responses. Cold I/R induces IRF-1 expression in hepatocytes that up-regulate death ligands (tumor necrosis factor receptor apoptosis-inducing ligand; FasL)/death receptor (DR5; Fas) in the liver. IRF-1–deficient livers express neither death ligands nor receptors and display reduced cold I/R.38 A recent report39 has revealed a critical role of IRF-1. IL15 is essential for development of T cells, NK cells, and NKT and is produced by hepatocytes upon IRF-1 induction through cold I/R. IRF-1 KO liver grafts are protected from cold I/R due to less induction of lymphocytes via IL15. Interestingly, administration of IL15/IL15Rα complexes partially restores cold IRI by increasing T cells, NK cells, and NKT.39
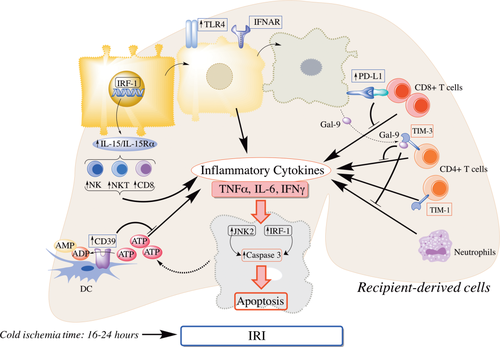
Molecular mechanisms implicated in liver IRI based on studies in the mouse liver transplant model. TLR4 and IFNAR are critical molecules involved in mouse liver transplant IRI. IRF-1 up-regulates IL15/IL15Ra production by hepatocytes that, in turn, activates donor-derived NK cells, NKTs, and CD8+ T cells to produce inflammatory cytokines. I/R-stressed hepatocytes release molecules such as ATP and Gal-9. CD39 on donor liver–derived DC hydrolyzes extracellular ATP and ameliorates liver injury. Gal-9 binds to TIM-3 on CD4+T cells and negatively regulates CD4+T cell activation and neutrophil infiltration, whereas TIM-1 on activated CD4+T cells promotes liver injury. Following IRI, hepatocytes up-regulated PD-L1 and induce apoptosis of infiltrating CD8+T cells, thus preventing exacerbation of IRI. IRF-1, along with JNK-2, is involved in inducing apoptosis of hepatocytes.
| Molecule | Significance Regarding IRI | Reference |
|---|---|---|
| IRF-1 | IRF-1–deficient livers express neither death ligands nor DRs and exhibit less cold I/R compared with WT livers | Ueki et al.38 (2010) |
| IRF-1 promotes liver transplant IRI via hepatocyte IL15/IL15Ra production | Yokota et al.39 (2015) | |
| TCA-3, CCL1 | Neutralization by anti-TCA-3 monoclonal antibody decreases neutrophil and T cell infiltration and attenuates cold IRI | Xie et al.37 (2006) |
| TLR-4 | TLR4 KO liver grafts are protected from cold IRI | Shen et al.41 (2007) |
| JNK-2 | JNK pathway induces mitochondrial depolarization and promotes hepatocyte death during cold I/R | Theruvath et al.40 (2008) |
| B7-H1 | Hepatocytes increase B7-H1 expression after cold I/R and induce T cell apoptosis in the graft | Ueki et al.44 (2011) |
| NTPDase-1 (CD39) | CD39 overexpression in liver grafts attenuates cold IRI CD39 deficiency exacerbates cold I/R | Pommey et al.42 (2013) Yoshida et al.43 (2013) |
| TIM-1, TIM-3 | Anti-TIM-1 antibody treatment abolishes neutrophil/macrophage activation and reduces cold IRI | Zhang et al.45 (2013) |
| TIM-3/Gal-9 activation protects livers from cold IRI | Liu et al.46 (2015) | |
| Type I IFN | Type I IFNAR KO grafts that lack type I IFN signaling show less cold IRI compared with WT grafts | Shen et al.47 (2012) |
| TNF-α | Donor liver TNFR1 protects against cold IRI, whereas recipient TNFR1 promotes cold IRI | Conzelmann et al.48 (2006) |
The c-Jun N-terminal kinase (JNK) signaling pathway is activated under conditions of stress and functions to enhance immune responses and apoptosis induction. During cold I/R, it induces mitochondrial depolarization and promotes hepatocyte death. JNK-deficient mouse liver grafts display reduced cold IRI, with less necrosis/apoptosis compared with WT grafts.40
Many other gene products, including Toll-like receptors (TLRs), other molecules expressed by immune cells, secreted cytokines, and chemokines, appear to regulate IRI in the mouse liver transplant model. TLR4 deficiency in donor grafts reduces neutrophil/lymphocyte infiltration after cold liver I/R through weak induction of chemokines and proinflammatory cytokines. On the other hand, TLR4 KO grafts show increased expression of hemoxygenase-1 (HO-1) that attenuates immune responses. Thus, TLR4 KO liver grafts are protected from cold IRI.41 The cell surface ectonucleotidase CD39, an ectonucleoside triphosphate diphosphohydrolase family member that hydrolyzes extracellular adenosine triphosphate (ATP) released from damaged or dead cells, can regulate inflammation. Its overexpression in liver grafts attenuates cold IRI,42 an effect also achieved by portal infusion of CD39-expressing liver DC.43 Moreover, CD39 deficiency in donor livers promotes inflammatory injury and allograft rejection.49 B7-H1 is also expressed on liver APCs and parenchymal cells, including hepatocytes. Hepatocytes increase B7-H1 expression after cold I/R and thus induce T cell apoptosis in the graft. Although WT livers that express B7-H1 during cold I/R are protected against excessive T cell attack, B7-H1 KO liver grafts fail to induce T cell apoptosis and exhibit more severe cold IRI.44
Type I IFNs (IFNα and IFNβ) produced by innate immune cells, including liver plasmacytoid DCs, are multifunctional cytokines that activate or regulate immune responses. Interferon receptor (IFNAR) KO grafts that lack type I IFN signaling cannot recruit neutrophils or macrophages and show reduced cold IRI. Interestingly, silencing HO-1 cancels the protective effect of IFNAR deficiency against cold IRI.47 TNF-α also plays an important role during tissue damage and inflammation. When tumor necrosis factor receptor-1 (TNFR1) KO livers are transplanted with cold preservation into WT recipients, they exhibit severe necrosis, apoptosis, and neutrophil infiltration. Interestingly, donor liver TNFR1 appears to protect against cold IRI, whereas host TNFR1 promotes cold IRI.48
T cell immunoglobulin and mucin (TIM)–1 is a member of the TIM domain family that is expressed on T cells and involved in T cell differentiation/activation. Cold I/R increases TIM-1 expression on activated CD4+ T cells within the liver. Blockade of TIM-1 signaling using mitochondrial antibodies abolishes neutrophil/macrophage activation, alters cytokine profiles toward T helper 2 cells, promotes Treg, and reduces cold liver IRI.45 TIM-3/galactin-9 (Gal-9) signaling regulates CD4+ immune responses and although a TIM-3 blockade enhances T helper 1 cell polarization and exacerbates hepatic cold IRI, TIM-3/Gal-9 activation protects mouse livers from cold IRI.46
Applications of Mouse Liver Transplantation to Other Aspects of Liver Immunobiology/Pathology
Origin of Liver Macrophages (Kupffer Cells) and the Immunologic Role of Toll-like Receptor 4 Expression and Antigen Presentation in the Liver
Using the mouse liver transplant model, Klein et al.,50 Polakos et al.,51 Klein et al.,52 and John et al.53 identified 2 distinct KC subsets in mouse liver: a radio-resistant, residual, non-BM-derived (sessile) population and a BM-derived KC population, by performing BM transplantation after sublethal irradiation. Only the BM-derived population was recruited into inflammatory foci in response to CD8+ T cell activation. To confirm this finding, liver transplantation was performed from congenic CD45.2 mice to CD45.1 recipients. As in the BM transplant experiment, a nearly equivalent distribution of BM-derived and sessile KCs were found 4 weeks after transplantation.52
The same group investigated the role of TLR4 expression in the liver by studying recipients of TLR4-deficient livers. These recipients received TCR transgenic (tg) OT-1 (CD8+) T cells and were primed with SIINFEKL (ovalbumin-derived peptide)–pulsed WT DCs. Six weeks later, SIINFEKL peptide was injected and clonal expansion of the OT-1 T cells determined. In recipients of TLR4-deficient livers, OT1 T cells were increased in the spleen, LNs, and liver compared with mice given WT grafts. Thus, TLR4 expression in the liver controls the systemic immune response by trapping activated CD8+ T cells, regulating circulating CD8+ T cell numbers, and down-modulating the extent of CD8+ T cell memory responses.53
To investigate the influence of restricted intrahepatic Ag presentation by hepatic cell types on immune reactivity, the same group transplanted livers from congenic B6.SJL mice (CD45.1 background, expressing MHC class I molecule Kb) into bm8 animals (CD45.2 background, expressing the Kbm8 mutation in the Kb molecule). To minimize the possibility of extrahepatic Ag presentation by donor-derived “passenger” leukocytes, BM chimeras were created as liver donors. Thus, B6.SJL mice were reconstituted with BM of the prospective liver transplant recipient strain, bm8. Using these chimeric liver grafts, only the graft parenchymal cells expressed the WT MHC class I molecule Kb and were competent to present the SIINFEKL peptide Ag. TCR tg CD8+ OT-I cells were adoptively transferred to the transplant recipients and SIINFEKL injected for 3 consecutive days. This restricted hepatic Ag presentation model was compared to control transplant recipients that expressed systemic Kb Ag. OT-I expansion was similar to or exceeded the expansion seen in control mice. Thus, Ag presentation in the liver was sufficient to promote activation and differentiation of CD8+ cells.50 Further investigation suggested that Ag-specific CD8+ T cells remain functionally competent after localization to the liver and retain their function after transfer or transplant. Investigation of naïve liver graft recipients indicated that those grafts that had previously experienced infection released trapped Ag-specific CD8+ T cells that functioned in a recall response.51
Small-for-Size Syndrome and Liver Regeneration
Use of partial liver grafts from either deceased or living donors expands the limited donor pool.54 However, insufficient liver mass may lead to small-for-size syndrome (SFSS), ie, liver failure characterized by coagulopathy, hyperbilirubinemia, and encephalopathy.54 To elucidate mechanisms underlying SFSS, a few groups have developed reduced-size liver transplantation in the mouse.55-58 Liver injury is worse with smaller graft size: 30% partial liver recipients have the most severe liver injury compared to 50% partial or whole liver transplantation 2 days after transplant.55, 56 Consistent with the extent of liver injury, 30% partial liver transplantation is associated with high mortality (100% within 4 days). By contrast, all mice that received 50% partial liver grafts survived for 100 days.56 The critical liver mass necessary for recipient survival and robust liver regeneration is estimated to be in the range 30%-50%, similar to observations in humans.55, 56
Investigations of the mechanisms underlying SFSS have revealed that KC inactivation with either gadolinium chloride or pentoxifylline reduces TNF-α signaling and liver injury, improving recipient survival and hepatic regeneration.57 Similar effects have been observed using TFNR1-/- mice, suggesting that KC-dependent TNF-α signaling plays a critical role in development of SFSS and liver regeneration after reduced-size liver transplantation.57
Amphiregulin (AR) is a ligand of the epidermal growth factor receptor and appears to promote liver regeneration. AR messenger RNA (mRNA) and protein expression are reduced significantly in 30% compared to 50% reduced-size liver grafts in association with increased TNF-α, IL6 mRNA expression, impaired liver regeneration, worse liver function, and decreased recipient survival.58 Exogenous AR partially reverses these effects, suggesting a therapeutic strategy to enhance regeneration in reduced-size grafts.
Role of the Liver in Pathogenesis of Liver Disease (Hemochromatosis, Fulminant Hepatitis)
There is controversy regarding the primary organ responsible for excess tissue iron deposition in hereditary hemochromatosis (HH). Hepcidin is the master regulator of systemic iron homeostasis produced by the liver and normally down-regulates the expression of ferroportin, the main iron exporter expressed in hepatocytes, duodenal enterocytes, and macrophages.59 In HH, a mutation in the hemochromatosis HFE gene causes abnormally low hepcidin levels, which up-regulates ferroportin and leads to high blood iron levels. When HFE WT livers are transplanted in HFE KO mice (HH phenotype), liver hepcidin mRNA expression, serum transferrin saturation, and liver iron content are normalized and iron content in the spleen and intestine improve.60 Conversely, transplantation of HFE KO livers into WT recipients results in the HH phenotype. These findings indicate that hepatic HFE and downstream hepcidin expression are crucial in the pathogenesis of HH.
Mouse liver transplantation has been used to determine the relative importance of hepatic versus systemic Fas (CD95) expression in the development of FH.61 Fas-mutant (MRL-lpr/lpr) liver transplantation into WT mice (MRL-lpr/lpr→WT) results in a Fas defect only in the liver, whereas WT liver transplantation into MRL-lpr/lpr mice (WT →MRL-lpr/lpr) results in a systemic Fas defect with Fas expression only in the liver. FH can be induced by administration of either an adenoviral vector containing FasL plasmid to promote FasL overexpression in the liver or by agonistic anti-Fas Ab. Overexpression of FasL in the liver results in severe hepatic injury, massive CD11b+ granulocyte infiltration, and increased apoptotic cells in all groups except nontransplanted MRL-lpr/lpr mice. Thus, FasL-induced FH occurs when Fas is expressed either in the liver or the host. However, agonistic anti-Fas-induced FH requires Fas expression in the liver. All graft recipients that received Fas-mutated liver grafts (both MRL-lpr/lpr→WT group and MRL-lpr/lpr→MRL-lpr/lpr) survived and exhibited no apoptosis or CD11b+ granulocyte infiltration. In contrast, all those that received WT liver grafts died within 48 hours and exhibited massive apoptosis and CD11b+ granulocyte infiltration. These results indicate that experimental FH involves not only direct Fas/FasL interaction but also the recruitment of inflammatory cells.
Acknowledgments
The authors thank Ms. Miriam Freeman for excellent administrative support and Dr. Angelica Perez-Gutierrez for assistance with the preparation of the figures and tables.