Living donor liver transplantation for intrahepatic arteriovenous fistula with hepatic artery reconstruction using the right gastroepiploic artery
Abbreviations
-
- CT
-
- computed tomography
-
- HA
-
- hepatic artery
-
- IHAVF
-
- intrahepatic arteriovenous fistula
-
- LDLT
-
- living donor liver transplantation
-
- LHA
-
- left hepatic artery
-
- LHV
-
- left hepatic vein
-
- MHV
-
- middle hepatic vein
-
- PV
-
- portal vein
-
- RGEA
-
- right gastroepiploic artery
Intrahepatic arteriovenous fistula (IHAVF), although extremely rare, is one of the indications for liver transplantation.1 A representative congenital cause for IHAVF is hereditary hemorrhagic teleangiectasia or Rendu-Osler-Weber disease,2 and the secondary causes are percutaneous puncture procedures such as liver biopsy.3 Hepatic artery reconstruction in patients with IHAVF is considered difficult because the hepatic arteries usually enlarge due to high-output arterial flow into the fistula.4 In living donor liver transplantation (LDLT), graft hepatic arteries are too thin to be directly anastomosed to these enlarged recipient hepatic arteries, and moreover, there is no vascular graft to be used as an interposition. Here, we report 2 cases of LDLT for IHAVF, in which hepatic artery reconstruction was accomplished using the recipient right gastroepiploic artery (RGEA).
Patient 1
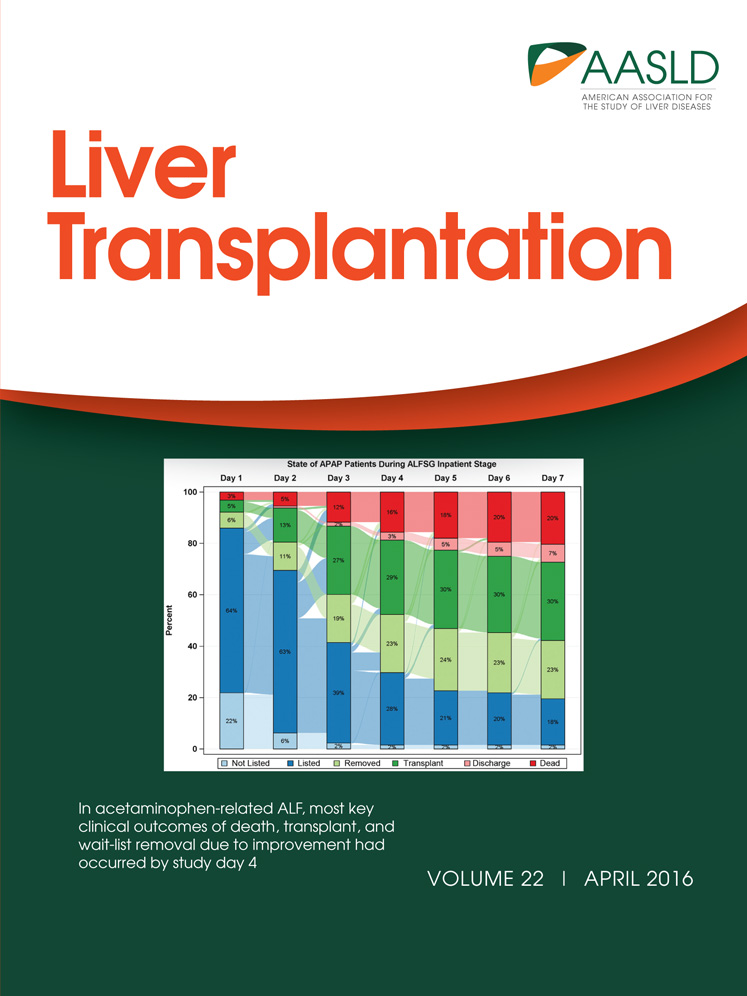
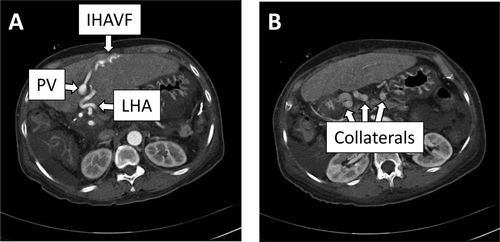
Patient 1 was a 57-year-old woman who had undergone LDLT using a left lobe graft for primary biliary cirrhosis 10 years earlier. She had a history of having undergone liver biopsy for abnormal liver function tests. She presented with abdominal pain and tarry stool. The laboratory test results indicated that she had severe anemia. Contrast-enhanced computed tomography (CT) revealed that there was an intrahepatic arterioportal fistula in the liver graft and the portal venous system was immediately enhanced by contrast in the early phase (Fig. 1). Upper endoscopy showed risky gastric varices but no obvious bleeding point. No apparent bleeding point could be detected by total colonoscopy. Capsule endoscopy finally detected multiple oozing points in the small intestine. These results indicated that severe portal hypertension led to the intestinal bleeding. Angiography confirmed the presence of an IHAVF between the intrahepatic segment 3 arteries and portal veins (PVs) and that the inflow hepatic artery was enormously enlarged. We tried to obstruct the IHAVF by interventional radiology to no avail, because of the multiple intrahepatic collateral arterial flows. Eventually, liver transplantation became the only option for curing the intestinal bleeding. Subemergency living donor liver retransplantation was performed using a left hepatic graft donated from her son 3 months after the presentation of abdominal pain and tarry stool. The pretransplant liver function tests are summarized in Table 1. Upon dissecting around the hepatic hilum, the enlarged hepatic artery whose diameter was approximately 1 cm was encountered, as preoperatively expected. Because of the size discrepancy between the graft and recipient hepatic arteries, it was impossible to directly anastomose these arteries. The hepatic vein and the PV were reconstructed in the usual manner. The recipient RGEA was freed from the greater curvature of the stomach by ligating the small branches into the stomach, and the RGEA (2 mm in outer diameter) was anastomosed to the graft left hepatic artery (LHA; 2 mm in outer diameter; Fig. 2A,B).5 Doppler ultrasound examination confirmed excellent arterial wave forms (Fig. 2C). The arterial flow measured by a flow meter was 144 mL/minute, which was considered sufficient in an LDLT with a left hepatic graft. The biliary reconstruction was accomplished by a hepaticojejunostomy. The postoperative course was uneventful without any ischemic conditions of the graft liver. For 6 years after the retransplantation, she had been constantly in a good condition. The contrast-enhanced CT taken on 3 years after the retransplantation showed the intact arterial flow from the recipient RGEA to the graft LHA. The current liver function tests (6 years after the retransplantation) of this patient were excellent (Table 1).
Contrast-enhanced CT of patient 1. (A) An IHAVF presumably caused by the liver biopsy was noted in segment 3. The LHA was tortuous and enlarged. The PV in the umbilical portion was immediately enhanced in the early phase. (B) Tortuous collaterals were noted around the stomach.
(A and B) Schematic diagrams of the preparation of the RGEA for hepatic artery reconstruction and (C) Doppler ultrasound wave forms of the intrahepatic hepatic artery, PV, LHV, and MHV of patient 1. (A) First, the dividing point of the RGEA was determined by measuring the distance between the stump of the LHA and the root of the RGEA so that there could remain some redundancy after anastomosis. (B) The RGEA was freed from the greater curvature of the stomach by dividing the omentum below the RGEA using a vessel sealing device and by ligating and dividing the small branches into the stomach. The root of the RGEA was clamped and then the RGEA was divided. The stump of the RGEA was brought up via the antegastric route without kinking of the artery. (C) Doppler ultrasound examination confirmed excellent arterial wave forms.
| Item | Normal Range | Patient 1 | Patient 2 | ||
|---|---|---|---|---|---|
| Preoperative | Current | Preoperative | Current | ||
| White blood cell (/μL) | 3300-8600 | 6740 | 6370 | 5810 | 7000 |
| Hemoglobin (g/dL) | 11.6-14.8 | 10.6 | 12.9 | 10.1 | 12.5 |
| Platelet × 103 (/μL) | 158-348 | 228 | 213 | 359 | 397 |
| Albumin (g/dL) | 4.1-5.1 | 2.6 | 3.9 | 2.9 | 3.9 |
| Total bilirubin (mg/dL) | 0.4-1.5 | 3.7 | 0.8 | 1.2 | 0.5 |
| Creatinine (mg/dL) | 0.46-0.79 | 0.74 | 0.71 | 0.44 | 0.57 |
| Aspartate aminotransferase (U/L) | 13-30 | 126 | 25 | 53 | 48 |
| Alanine aminotransferase (U/L) | 7-23 | 83 | 24 | 45 | 37 |
| Alkaline phosphatase (U/L) | 106-322 | 504 | 341 | 1901 | 645 |
| Prothrombin time–international normalized ratio | 0.90-1.10 | 1.00 | Not determined | 1.28 | Not determined |
Patient 2
Patient 2 was a 43-year-old female. She had been suffering from multiple intrahepatic secondary biliary stenoses caused by multiple arteriovenous malformations for 6 years before the transplantation. Interventional biliary stenting was initially effective for relieving the biliary strictures. Cholangitis and intrahepatic abscesses, however, had gradually become refractory. Contrast-enhanced CT revealed that there were enormously enlarged hepatic arteries (Fig. 3). Then, she had begun to complain of exertional dyspnea caused by high-output cardiac failure approximately 3 years before the transplantation. There was no detectable arteriovenous malformation other than the liver. Liver transplantation was the last resort against these deteriorating conditions. Her husband wished to donate his partial liver, and the patient was referred to our hospital to undergo LDLT. Preoperative 3D-CT of the donor revealed that the left hepatic graft would have 2 small hepatic arteries (the left and middle hepatic arteries). The pretransplant liver function tests are summarized in Table 1. On entering the peritoneal cavity, tangled, enormously enlarged hepatic arteries were encountered in the hepatoduodenal ligament. These arteries together with the extrahepatic bile duct were divided at the hepatic hilum using an autosuture device. The hepatic vein and the PV were reconstructed in the usual manner. The hepatofugal portal venous flow spontaneously returned to hepatopetal one after reperfusion. The recipient RGEA was freed from the greater curvature of the stomach by ligating the small branches into the stomach, and the RGEA (2 mm in outer diameter) was anastomosed to the graft LHA (2 mm in outer diameter). The arterial flow measured by a flow meter was 88 mL/minute, which was considered adequate in an LDLT with a left hepatic graft. The graft middle hepatic artery (1.5 mm in diameter) was ligated after confirmation that there was sufficient backflow from the stump of the middle hepatic artery. Biliary reconstruction was done by a hepaticojejunostomy. Her postoperative course was uneventful without any ischemic conditions of the graft liver. For 6 months after the transplantation, she had been in a good condition. The contrast-enhanced CT taken 1 month after the transplantation showed the intact arterial flow from the recipient RGEA to the graft LHA. The current liver function tests (6 months after the transplantation) were satisfactory (Table 1).
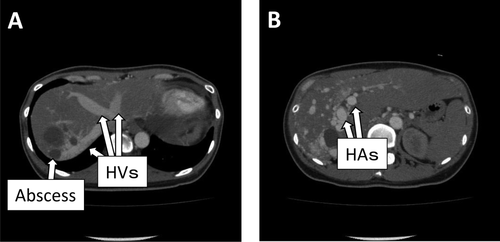
Contrast-enhanced CT of patient 2. (A) The intrahepatic veins were vigorously enhanced even in the very early phase of contrast-enhanced CT. (B) The hepatic arteries enormously enlarged to nearly the size of the aorta.
In considering LDLT for IHAVF, the most important fact that surgeons have to pay attention to is that the recipient hepatic arteries have to be enormously enlarged to be directly anastomosed to the graft hepatic arteries. The hepatic arteries in patients with IHAVF have excessive blood flow because they are directly connected to low-pressure venous systems. As a result, the hepatic arteries gradually enlarge, and by the time liver transplantation is considered, they become too large to be directly anastomosed to tiny graft arteries. Unlike deceased liver transplantation, the graft hepatic arteries to be reconstructed in LDLT were too thin to be directly anastomosed to those huge hepatic arteries in patients with IHAVF, and there is usually no interposition vascular graft obtained from deceased donors. The usefulness of the recipient RGEA for hepatic artery reconstruction has been reported by many investigators.5 The RGEA has great flexibility compared to other visceral arteries such as the left gastric artery or the splenic artery. The normal RGEAs, however, are usually thin and their diameters are approximately less than 2 mm. Surgeons may think that the arterial flow of the RGEA is not sufficient to provide adequate arterial blood through the entire hepatic graft. However, there have not been any reports suggesting ischemic conditions related to hepatic artery reconstruction by the RGEA although the number of patients with such reconstruction was small. Most patients with IHAVF have undergone multiple sessions of interventional therapy because certain portions of IHAVFs are interventionally treated. As a result, the inflow hepatic artery may be injured and may not be suitable for an inflow artery for hepatic artery reconstruction. Hepatic artery reconstruction using the left gastric artery or the splenic artery was reported with success.4, 5 However, it is generally difficult to dissect the left gastric artery free from the lessor curvature of the stomach due to the massive collateral vessels. Moreover, it is not always possible to obtain enough length of the left gastric artery so as to be directly anastomosed to the graft artery. On the other hand, dissecting the splenic artery from the retroperitoneum is far more difficult.
We determined to use a left graft in the 2 patients although they were an adult-to-adult LDLT. Generally, patients with IHAVF who need liver transplantation have relatively good hepatic reserve. Therefore, a small left hepatic graft can be selected in these patients. At this point, a question arises. Can the recipient RGEA be used safely even in LDLT using a larger right hepatic graft? We strongly believe the arterial flow supplied by the RGEA can at least maintain the graft viability as long as the anastomosis remains intact.
From these points of view, the RGEA can be the first choice for an inflow artery in recipients with IHAVF. Surgeons should ascertain that there is the intact RGEA in the recipient during preoperative evaluation for patients with IHAVF.
-
Hideaki Uchiyama, M.D., Ph.D.
-
Ken Shirabe, M.D., Ph.D.
-
Tomoharu Yoshizumi, M.D., Ph.D.
-
Toru Ikegami, M.D.
-
Norifumi Harimoto, M.D., Ph.D.
-
Shinji Itoh, M.D.
-
Koichi Kimura, M.D., Ph.D.
-
Hirohisa Okabe, M.D., Ph.D.
-
Yoshihiko Maehara, M.D.
-
Department of Surgery and Science
-
Graduate School of Medical Sciences
-
Kyushu University
-
Fukuoka, Japan