Flushing the liver with urokinase before transplantation does not prevent nonanastomotic biliary strictures
Potential conflict of interest: Nothing to report.
Abstract
The aim of the present study was to assess whether flushing the donor liver with urokinase immediately before implantation reduces the incidence of nonanastomotic biliary strictures (NASs) after liver transplantation, without causing increased blood loss, analyzed as a historical cohort study. Between January 2005 and October 2012, all liver (re-)transplantations were included. Of the 185 liver transplant recipients included, 63 donor livers between January 2010 and October 2012 received urokinase (study group), whereas the donor liver of 122 consecutive recipients, who served as a historical control group, between January 2005 and January 2010 did not receive urokinase. Basic donor (Eurotransplant donor risk index) and recipient (age, body mass index, laboratory Model for End-Stage Liver Disease score) characteristics did not significantly differ in both groups. Thirty-three recipients developed NASs: 22 in the control group (18%) and 11 (17.5%) in the study group (P = 0.68). Analyzed separately for donation after circulatory death (P = 0.42) or donation after brain death (P = 0.89), there was no difference between the groups in incidence of NAS. Of all the recipients developing NAS, 7 (21%) needed retransplantation and all others were treated conservatively. Autologous blood transfusion requirements did not differ significantly between both groups (P = 0.91), whereas interestingly, more heterologous blood transfusions were needed in the control group (P < 0.001). This study has its limitations by its retrospective character. A multi-institutional prospective study could clarify this issue. In conclusion, arterial flushing of the liver with urokinase immediately before implantation did not lead to a lower incidence of NAS in this study, nor did it lead to increased blood loss. Liver Transplantation 22 420-426 2016 AASLD
Abbreviations
-
- aPTT
-
- activated partial thromboplastin time
-
- BMI
-
- body mass index
-
- CIT
-
- cold ischemia time
-
- DBD
-
- donation after brain death
-
- DCD
-
- donation after circulatory death
-
- ET-DRI
-
- Eurotransplant donor risk index
-
- FWIT
-
- first warm ischemia time
-
- HAT
-
- hepatic artery thrombosis
-
- HTK
-
- histidine tryptophan ketoglutarate
-
- labMELD
-
- laboratory Model for End-Stage Liver Disease
-
- NAS
-
- nonanastomotic biliary stricture
-
- PTT
-
- partial thromboplastin time
-
- RBC
-
- red blood cell
-
- SD
-
- standard deviation
-
- tPA
-
- tissue plasminogen activator
-
- WIT
-
- warm ischemia time
Biliary complications are a well-known, major cause of morbidity and graft failure in recipients after liver transplantation.1, 2 The most troublesome are the so-called nonanastomotic biliary strictures (NASs), with an incidence of 5%-15% reported in most current studies,3, 4 and in up to 30% of patients receiving a liver from donation after circulatory death (DCD).5 With direct treatment of strictures, by using endoscopic or percutaneous cholangiographic dilatations and stenting, more than 50% of patients with NASs can be treated successfully.6-11
The pathophysiology of NAS development still remains unknown. Over the years, several risk factors have been indicated, suggesting that its origin may be multifactorial. In addition to immunological injury and bile salt–induced injury, it is suggested that ischemia injury to the peribiliary vascular plexus plays a critical role.12 During the donor procedure, the peribiliary arterial plexus may not be completely flushed out. Because the blood supply to the biliary tract is solely dependent on arterial inflow, these microcirculatory disturbances in the peribiliary plexus may lead to obstruction and may subsequently result in insufficient bile duct preservation.13, 14
Three previous studies with historical controls suggest that adding a thrombolytic agent, such as urokinase or tissue plasminogen activator (tPA) to the preservation fluid (after trimming of the donor liver, on the back table, or before completion of the portal vein anastomosis), seems to reduce the incidence of NAS. The hypothesis was that this might be the result from dissolving microthrombi in the microvascular system of the biliary tree.15-17
The aim of the present study was to assess whether flushing the donor liver with urokinase directly before transplantation reduces the incidence of NAS without causing increased blood loss.
Patients and Methods
Between January 2005 and October 2012, all orthotopic liver transplantations at the Leiden University Medical Center (Leiden, the Netherlands) were included in this study.
Exclusion criteria were domino, split, or auxiliary liver transplantations. Clinical information was obtained from a prospectively collected database. Covariates included donor demographics, recipient demographics, pretransplant information, intraoperative data, and postoperative outcomes. Calculated laboratory Model for End-Stage Liver Disease (labMELD) scores were included in the recipient analysis.
The labMELD score was calculated using laboratory data (creatinine, bilirubin, international normalized ratio) and did not include exception points that were given for liver malignancies or other medical conditions.
On the basis of existing literature, a protocol change was made as of January 2010 to flush the donor liver with urokinase, directly before transplantation. This protocol change was approved by the institutional ethics committee.
Definition of NAS
NAS was defined as described by Ten Hove et al.18 NAS was any stricture or irregularity of the intrahepatic or extrahepatic bile ducts of the liver graft that was at least 1 cm above the anastomosis, with or without dilation and with or without biliary sludge formation, and treated endoscopically with endoscopic retrograde cholangiopancreatography and dilation and/or stenting, percutaneously with percutaneous transhepatic cholangiography and biliary drainage or by surgical intervention. Therefore, all these NASs were clinically significant. Hepatic artery thrombosis (HAT) by either Doppler ultrasound, or conventional angiography, as well as isolated strictures/stenosis at the bile duct anastomosis and related dilations were, by definition, excluded from analysis.
Operative Techniques
The procurement of organs was performed as described by the Eurotransplant protocol. During procurement, the donor liver was flushed with preservation fluid under a pressure of 300 mm Hg (the type of perfusion fluid used depended on the country where the procurement took place within the Eurotransplant region). During procurement in DCD liver allografts, 5000 IU of heparin was administered during initial organ perfusion. In donation after brain death (DBD) liver allografts, 300 IU/kg of heparin was administered 5 minutes before cross-clamping. After procurement, the liver was sent to our hospital. Since January 1, 2010, as a change in center protocol, after inspection of the donor liver and immediately before implantation, the hepatic artery was flushed with 250,000 IU of manually pressurized urokinase on the back table. Hereafter, the hepatic artery was clamped to prevent backflow. After a minimum period of 10 minutes after flushing with urokinase, the hepatic artery was flushed with 500 mL of preservation fluid (histidine tryptophan ketoglutarate [HTK] or University of Wisconsin) in order to prevent systemic introduction of urokinase. Also, according to standardized protocol, the portal vein was flushed with 150 mL of albumin during caval anastomosis in order to prevent systemic introduction of urokinase. Further implantation of the liver allograft was done according to protocol. Before January 1, 2010, the same protocol was carried out, only without administration of urokinase. After consultation of the medical ethics committee, recipients did not have to give informed consent because the administration of urokinase was implemented as a new center protocol.
Statistical Analysis
Continuous variables were presented as median (range) and standard deviation, whereas categorical variables were presented as number and percentage. Patient and graft survival curves and the cumulative incidence of NAS were calculated using the Kaplan-Meier method and compared using the log-rank test. Categorical variables were compared with the Pearson's chi-square test or Fisher's exact test, where appropriate. Characteristics of the donor, transplantation, and recipient were analyzed using the 2-tailed Student t test. Blood loss was analyzed using the Mann-Whitney U test. The level of significance was set at 0.05. Statistical analyses were performed using SPSS software version 22.0 for Windows (SPSS Inc., Chicago, IL).
Power Analysis
With an anticipated reduction of NAS from 45% to 10% on the basis of previous studies on DCD liver transplantation,15-17 the power of this study would be 83.2% when comparing 28 DCD livers in the study group to 17 DCD livers in the control group.
With an anticipated reduction of NAS from 20% to 5% on the basis of previous studies concerning DBD liver transplantation, the power of this study would be 80.1% when comparing 94 DBD livers in the study group to 46 DBD livers in the control group.
Results
Of the 205 patients who received a liver transplantation between January 2005 and October 2012, 5 recipients were excluded based on missing information on receiving urokinase, 3 recipients were excluded based on protocol deviation (Fig. 1). Of the 197 liver recipients remaining for the study, 127 donor livers did not receive urokinase (historic control group), and 70 donor livers received urokinase (study group).
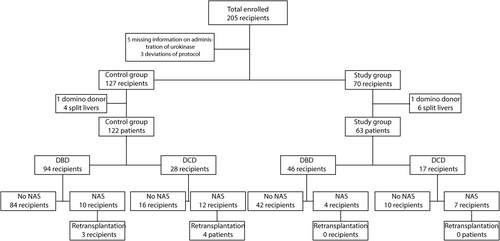
Study design.
In the historic control group, 5 recipients were excluded (4 split-liver transplantations, 1 domino donor), leaving 122 recipients in this group. In the study group, 7 recipients were excluded (6 split-liver transplantations, 1 domino donor), leaving 63 recipients.
Donor and Recipient Characteristics
Table 1 shows the basic donor and recipient characteristics of both groups. The mean Eurotransplant donor risk index (ET-DRI)19 in the control group was 1.8 ± 0.3 (range, 1-3.1), in the study group 1.8 ± 0.4 (range, 1-2.6; P = 0.56). Of 3 donors, the ET-DRI could not be calculated. Of the donors in the control group, 51% were female versus 48% in the study group (P = 0.76). Donor body mass index was lower in the control group than in the study group. The mean cold ischemia time (CIT) of the transplanted livers in the control group was 572 ± 142 minutes (224-1090 minutes), in the study group 535 ± 129 minutes (range, 230-850 minutes; P = 0.09). The mean first warm ischemia time in the control group was 16.7 ± 5 minutes (range, 11-31 minutes), in the study group 17.6 ± 6 minutes (range, 9-31 minutes; P = 0.60). The mean labMELD score in the control group was 16.6 ± 8.7 (range, 6-40), in the study group 16.6 ± 8.9 (range, 6-40; P = 0.99).
| Urokinase Group (n = 63) | Controls (n = 122) | P Value | |
|---|---|---|---|
| ET-DRI | 1.8 ± 0.3 | 1.8 ± 0.3 | 0.56 |
| Donor age, years | 49.4 ± 15.0 | 46.9 ± 14.2 | 0.27 |
| Donor BMI, kg/m2 | 25.2 ± 3.3 | 24.0 ± 3.3 | 0.03 |
| FWIT, minutes | 17.6 ± 6.0 | 16.7 ± 4.5 | 0.6 |
| CIT, minutes | 535.0 ± 129.0 | 572.0 ± 142.2 | 0.09 |
| Recipient WIT, minutes | 35.9 ± 8.5 | 33.8 ± 8.4 | 0.12 |
| Recipient age, years | 51.9 ± 11.6 | 52.3 ± 10.8 | 0.80 |
| Recipient BMI, kg/m2 | 25.8 ± 4.6 | 26.2 ± 4.8 | 0.53 |
| LabMELD | 16.6 ± 8.9 | 16.6 ± 8.7 | 0.99 |
| Time to NAS diagnosis, days | 119 ± 202 | 172 ± 363 | |
| Time of follow-up, days | 731 ± 495 | 1731 ± 1049 |
- NOTE: Data are presented as mean ± SD. Time to NAS diagnosis and time of follow-up are presented as median ± SD.
Biliary Complications
In total, 33 (17.8%) recipients developed NASs, of which 22 (18%) recipients were in the control group, and 11 (17.5%) recipients were in the study group. None of the recipients had evidence of HAT or stenosis. The mean follow-up in the control group was 1543 ± 1049 days (range, 1-3278 days) versus 675 ± 495 days (range, 1-1434 days) in the study group. The median number of days of follow-up was 1731 ± 1049 days (range, 312-2356 days) in the study group versus 731 ± 495 days (range, 119-1109 days) in the control group (Table 1).
In the control group, the mean number of days until NAS was diagnosed was 295 ± 363 days (range, 22-1454 days). In the study group, the mean number of days was 189 ± 202 days (range, 30-723 days; P = 0.38). Graft survival, censored for death, shows equal results for both groups (P = 0.68; Fig. 2). In the control group, the median number of days until NAS was diagnosed was 172 ± 363 days (range, 71-346) compared to 119 ± 202 days (range, 48-216) in the study group.
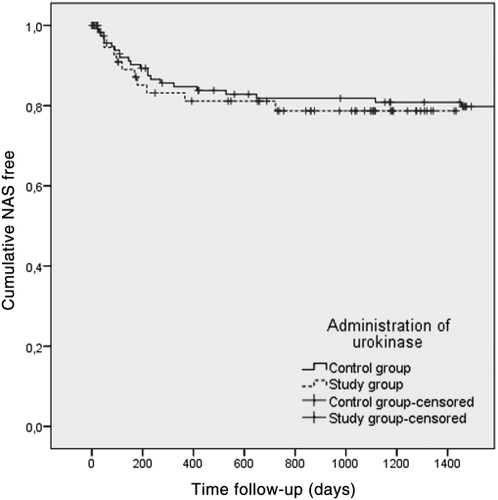
Graft survival, censored for death.
Comparison of liver transplantations from DCD donors only also showed equal graft survival; 7 (41%) recipients in the study group developed NAS versus 12 (43%) recipients in the control group (P = 0.42).
In the control group, 10 (11%) recipients who received a liver allograft from DBD donors developed NAS versus 4 (9%) recipients in the study group. This was not different (P = 0.89). Of all cases, 7 (21%) recipients needed retransplantation for NAS.
Postreperfusion Blood Loss
Table 2 shows the hematological and coagulation parameters of both groups preoperative; the activated partial thromboplastin time (aPTT) values during anhepatic phase, after reperfusion, and after surgery; the number of packed red blood cells (RBCs) transfused during the first 24 hours from incision; and volume of autologous blood transfused.
| Urokinase Group (n = 63) | Controls (n = 122) | P Value | |
|---|---|---|---|
| Platelet count before surgery, ×109/L | 128 ± 96 | 118 ± 90 | 0.47 |
| PTT before surgery, seconds | 20 ± 8 | 17 ± 5 | <0.01 |
| Fibrinogen before surgery, g/L | 3.1 ± 1.7 | 3.1 ± 1.7 | 0.97 |
| aPTT before surgery, seconds | 39 ± 8 | 35 ± 8 | <0.01 |
| aPTT anhepatic phase, seconds | 45 ± 15 | 43 ± 11 | 0.27 |
| aPTT after reperfusion, seconds | 70 ± 23 | 72 ± 28 | 0.57 |
| aPTT after surgery, seconds | 51 ± 16 | 51 ± 18 | 0.92 |
| Packet cells, units | 5.6 ± 6.2 | 8.6 ± 7.7 | <0.01 |
| Cell saver, mL | 946 ± 1166 | 919 ± 1320 | 0.91 |
- NOTE: Data are presented as mean ± SD.
Most remarkably, the mean preoperative aPTT in the control group was 34.5 ± 7.5 versus 38.5 ± 7.5 seconds in the study group (P ≤ 0.01), whereas aPTT did not differ between the study group and control group in the anhepatic phase after reperfusion and after surgery.
The mean packed RBCs transfused in the control group was 8.6 ± 7.7 versus 5.6 ± 6.2 units in the study group (P < 0.01). The mean volume of autologous blood transfused in the control group was 919 ± 1320 versus 946 ± 1166 mL in the study group (P = 0.91).
Discussion
The present retrospective cohort study demonstrates that flushing the donor liver with urokinase immediately before liver transplantation is safe but does not prevent the development of NAS. It also did not lead to an increase of transfusion requirements or disturbed clotting.
In contrast to previous studies15-17 describing a decrease in the rate of NAS after thrombolysis before liver transplantation, this study could not find a decrease in NAS rate. The first study by Hashimoto et al.15 was a retrospective study, with the injection of tPA in the donor hepatic artery on the back table in DCD liver transplantations, resulting in significantly less NAS development without increased blood loss. Lang et al.16 described a prospective study with double perfusion of the donor liver with urokinase in DCD liver transplantation, resulting in significantly less NAS after 1 year of follow-up. Seal et al.17 described a retrospective analysis of DCD liver transplantations with an intraoperative tPA injection, which minimized the incidence of NAS without increasing the need of intraoperative blood transfusion. When looking closely at the dosage, type, and timing of the thrombolytic agent, our study has some minor differences compared to the studies previously published. In the study by Hashimoto et al.,15 heparin was given to the donor before withdrawal of life support, and the liver allograft was perfused on the back table with 0.5 mg/100 g graft tPA in the donor hepatic artery. Lang et al.16 perfused the arterial system of the donor liver twice. First, they used a dosage of 2000 mL HTK solution that contained 2 MU urokinase for perfusion through the arterial system. After trimming of the donor liver, the arterial system was perfused again with 1 MU urokinase. In the study by Seal et al.,17 heparin was given to the donor before withdrawal of life support and, based on donor's weight and before completion of the portal vein anastomosis, 100 mg/kg tPA was perfused in the donor liver to account for variations in graft size.15-17 In our study, the donor liver was perfused through the hepatic artery after inspection on the back table, with a high fixed dose of 250,000 IU of urokinase, manually pressurized, in order to dissolve possible microthrombi.
To our knowledge, no previous studies have been published that show superiority for tPA, compared to urokinase, in low temperature circumstances.
Furthermore, the CIT in the study by Hashimoto et al.15 and Seal et al.17 was much shorter. They described a CIT of 422 ± 96 minutes and 5.1 ± 1.2 hours, whereas the CIT in this study was 572 ± 142 minutes in the control group and 535 ± 129 minutes in the study group. Lang et al.16 described a CIT between 2 and 13.5 hours but did not mention a mean value.
The effect of thrombolytic agents has been reported in the experimental as well as clinical transplantation of various organs from DCD donors,20-23 suggesting that pretreatment with thrombolytic agents could be helpful in human liver transplantation. A possible explanation for not seeing a decrease in the rate of NAS in the current study could be the timing of the intervention. The therapeutic principle of administrating urokinase is to dissolve possible microthrombi from the peribiliary microcirculation. It may be that administrating urokinase immediately after organ procurement may be beneficial, whereas late administration of urokinase may not be able to prevent or dissolve microthrombosis. However, this hypothesis is not supported by Seal et al.,17 who administered tPA before completion of the portal vein anastomosis in order to limit the effects of hypothermia and dilution. The administration of urokinase in the donor liver is not part of the liver transplantation protocol in many other centers in the Eurotransplant region. For that reason, we were not able to administer urokinase during the donation procedure.
The absence of microthrombi in the microvascular system of the biliary tree also has to be considered as a possible explanation why urokinase in our hands did not prevent NAS. In a study by op den Dries et al.,24 biopsies were taken from the donor bile duct in 128 liver transplant procedures. In the peribiliary plexus, thrombi were found in only 2.7% of these bile ducts from the livers that developed NAS, suggesting that thrombosis may not play a critical role in the development of NAS.
In a study by Hansen et al.,25 histological evaluations of 93 donor common bile ducts, received after recirculation of the hepatic artery and before biliary end-to-end anastomosis in LT, were performed. With regard to NAS, they found that necrosis of the bile duct wall, arteriolonecrosis, vascular lesions, and intramural bleeding were statistically relevant associated factors. Thrombosis was not of statistical influence on occurrence of NAS. This also supports the theory that thrombosis does not play a critical role in the development of NAS.
Furthermore, Burlage et al.26 believed that other factors, such as increased experience, explain the observed differences found in the study by Seal et al.17
The use of allogeneic and autologous blood as a measurement of blood loss has been described before by Hendriks et al.27 Surprisingly, in this study, there was significantly more need for packed RBC transfusion in the control group, even though this group had significantly better coagulation parameters preoperatively. A possible explanation for this may be a positive effect of increasing experience within the transplantation team, as has been described previously.27, 28 During the study period, the transfusion protocol has not changed.
The extent and severity of NAS after liver transplantation determines its prognosis and management. Diffuse strictures have worse prognosis than local strictures because of a lack of therapeutic options. Even though we did not see a significant difference in the rate of NAS, the number of retransplantations due to NAS in the first year after transplantation could potentially show a difference because of different severity of NAS. Of all cases of NAS in the control group, 6 (27%) recipients required retransplantation in the first year after liver transplantation. Five (23%) recipients received a retransplantation in the first year after liver transplantation and 1 (5%) recipient was placed on the waiting list for retransplantation. In the study group, none of the recipients received a retransplantation in the first year after liver transplantation, and 1 (9%) recipient was placed on the waiting list for retransplantation. This difference was not significant (P = 0.32). All other cases of NAS were treated conservatively by using balloon dilatation of the bile duct combined with the placement of an intraductal stent. The median number of interventions for NAS was 5.0 ± 2.4 (range, 2.0-7.0) in the control group versus 4.5 ± 4.2 (range, 2.0-8.0) in the study group.
Even though the median number of days of follow-up is shorter in the study group, compared to the control group, we do not believe this difference had an influence on the outcome because the median number of days until NAS diagnosis in both groups (172 ± 363 days [range, 71-346 days] in the control group versus 119 ± 202 days [range, 48-216 days] in the study group) is much shorter compared to the median number of days of follow-up in both groups (1731 ± 1049 days in the study group versus 731 ± 495 days in the control group). Because NAS is more common after DCD liver transplantation, compared to DBD liver transplantation, both groups were analyzed separately. When analyzed separately, no difference was found in the incidence of NAS after DCD liver transplantation (P = 0.42). Also, no difference was found in the incidence of NAS after DBD liver transplantation (P = 0.89).
This study has its limitations by its retrospective character. A multi-institutional prospective study could clarify this issue.
To conclude, arterial flushing of the donor liver with urokinase immediately before implantation did not lead to a lower incidence of NAS in this study, nor did it lead to increased blood loss.