Incidence and diagnosis of active toxoplasma infection among liver transplant recipients in Western Turkey
Abstract
Toxoplasmosis is a serious and potentially life-threatening disease in liver transplant recipients while they are immunosuppressed. We report the clinical and laboratory findings related to active toxoplasma infection associated with 40 immunosuppressed liver transplant procedures that took place over a 12-month period at a major transplant unit in Izmir, Turkey. Twenty-seven (67.5%) of the 40 transplant recipients were found to be seropositive for toxoplasma infection and therefore at risk of reactivated infection. From the serological status of the donors, which was ascertained in 38 of 40 cases, we identified 3 (7.9%) of 38 transplants to be from a seropositive donor to a seronegative recipient. In 10 (26.3%) of 38 transplants, both the donor and recipient were seronegative, and this excluded toxoplasma as a risk. A comparison of real-time polymerase chain reaction (PCR) and nested PCR was undertaken in combination with a range of serological assays (the Sabin-Feldman dye test, enzyme immunoassay immunoglobulin M, and immunosorbent agglutination assay immunoglobulin M). Ethylene diamine tetraacetic acid blood samples from 3 of the 30 recipients at risk from toxoplasma were found positive by PCR, but only 1 of these was found positive in both assays. Among the 3 PCR-positive patients, immunoglobulin M and immunoglobulin G antibody levels increased in only 1 patient. Correlations between symptoms, laboratory findings, and clinical management (use of anti-toxoplasma therapy) are presented. Our findings suggest that toxoplasma presents a significant risk to our liver transplant population and that PCR is a helpful addition in identifying active infections and hence in informing clinical management decisions. Liver Transpl 14:1526–1532, 2008. © 2008 AASLD.
Toxoplasma gondii is one of the most successful protozoan parasites because it has a very broad host range, infecting all warm-blooded animals, including humans.1 Toxoplasmosis can occur following the reactivation of previously latent bradyzoite tissue cysts in transplant recipients under intense immunosuppressive treatment and may cause severe or life-threatening disease.1-6 As the number of transplants performed in the world continues to increase, the burden of disease associated with the reactivation of toxoplasmosis has also increased.5, 7 The mortality and morbidity rates of toxoplasmosis in liver transplant recipients are high because of late diagnosis and delayed initiation of treatment.8, 9
Serological assays are useful for identifying patients at risk before transplantation,10, 11 but they are of limited value in diagnosing reactivated infection because profound immunosuppressive treatment can result in a decrease in the total antibody amount due to leukoneutropenia.2, 10, 12-14 Currently, the diagnosis of active toxoplasma infection in immunosuppressed patients is based principally on the detection of DNA by polymerase chain reaction (PCR) because of the relatively high sensitivity and specificity of this method2, 9, 12, 15-19; nested PCR is a particularly good method.15, 20 More recently, quantitative real-time PCR has been employed to assess changes in the levels of T. gondii DNA in order to monitor the efficacy of treatment.17, 18, 21-23 Although toxoplasmosis has been reported to be uncommon in liver transplant recipients, there is only limited evidence addressing the rate of transplant-mediated transmission.2, 5, 6, 14 Thus, the present study was designed to assess the rate of primary or reactivated toxoplasmosis associated with liver transplantation. This study also compared the results obtained from a range of available laboratory tests [real-time PCR, nested PCR, Sabin-Feldman dye test, enzyme immunoassay (EIA) immunoglobulin M (IgM), and immunosorbent agglutination assay (ISAGA) IgM] in order to assess optimal investigation pathways. These assessments were based on the investigation for 4 months of a cohort of liver transplant recipients who were receiving immunosuppression.
Abbreviations
AB, antibiotic treatment to prevent bacterial infection; bp, base pairs; CS, corticosteroid; EIA, enzyme immunoassay; F, fever; G, gentamicin ophthalmic drop; H, headache; IgG, immunoglobulin G; IgM, immunoglobulin M; ISAGA, immunosorbent agglutination assay; N, negative; Na, nausea; nt, nucleotides; P, positive; PBS, phosphate-buffered saline; PBS-T, phosphate-buffered saline (pH 7.4) containing 0.5% Tween-20; PCR, polymerase chain reaction; SF, Sabin-Feldman; TMP-SMX, trimethoprim-sulfamethoxazole; VD, visual disturbances.
PATIENTS AND METHODS
Patient Samples
Blood samples were collected from 40 liver transplant recipients and their donors that were admitted to the Transplantation Unit of the Department of General Surgery of Ege University Medical School between May 2005 and May 2006. The recipients and donors were grouped on the basis of the initial serological assays (Table 1). In group 1 (10 cases), both the recipient and donor were found to be seronegative on initial screening, and there was no further investigation because of no risk of transmission during liver transplantation. In the remaining 30 cases, under the risk of primary or reactivated toxoplasmosis associated with liver transplantation, blood samples were collected from the recipients 7 days prior to transplantation and 7, 17, 27, 34, 44, 54, 64, 90, and 120 days after transplantation. In addition, 1 blood sample was collected from the donors before transplantation. Patients that were at risk of toxoplasmosis were selected according to their T. gondii–specific IgM and immunoglobulin G (IgG) results (that is, serum samples of recipients and donors that were negative were excluded from the study group). In order to assess the specificity of the PCR and serological assays, 2 control groups were included: control group 1, which had 10 patients with leishmaniasis and malaria, and control group 2, which had 10 Toxoplasma-seronegative healthy individuals. The experimental plan was performed under the instructions and approval of the Research Ethics Committee of the Ege University Medical School.
| Liver Transplantation Cases | |
|---|---|
| Group 1 (D−/R−) | 10 |
| Group 2 (D+/R−) | 3 |
| Group 3 (D−/R+) | 4 |
| Group 4 (D+/R+) | 21 |
| Group 5 (D?/R+)* | 2 |
| Total | 40 |
- Abbreviations: +, seropositive; −, seronegative; ?, serology unknown; D, donor; R, recipient.
- * The serology was not determined in the cadaver donor transplantation group.
DNA Extraction and PCR Analysis
Isolation of DNA from blood samples of patients and donors was performed with the High Pure PCR Template Preparation Kit according to the manufacturer's protocol (Roche Applied Sciences, Germany). At the end of the procedure, 200 μL of eluted buffer yielded approximately 0.5 to 25 ng/μL purified DNA. Nested PCR, targeting the B1 gene of T. gondii (GenBank no. AF179871), was performed as described.15, 20 Briefly, the first set of primers, used to amplify the 287–base pair (bp) gene fragment in the nested PCR reaction, were 5′-TCAAGCAGCGTATTGTCGAG-3′ [20 nucleotides (nt), oligo 1, forward primer] and 5′-CCGCAGCGACTTCTATCTCT-3′ (20 nt, oligo 2, reverse primer); the second set of primers, used to amplify the 194-bp gene fragment in the second nested PCR reaction, were 5′-GGAACTGCATCCGTTCATGAG-3′ (21 nt, N1, forward primer) and 5′-TCTTTAAAGCGTTCGTGGTC-3′ (20 nt, C1, reverse primer). The first 50-μL final volume of the amplification reaction included 25 μL of the purified DNA template, 1 μM primers (oligo 1 and oligo 2), 1.25 U of GoTaq DNA polymerase (5 U/μL; Promega, United States), 1× GoTaq reaction buffer (Promega), and 0.2 mM deoxyribonucleotide triphosphate (Invitrogen, United States). The PCR amplification reaction was performed with the following calculated control protocol: a 30-second initial denaturation step at 94°C followed by 50 cycles of 15 seconds at 94°C, 30 seconds at 45°C, and 45 seconds at 72°C and a final extension of 10 minutes at 72°C. The second 50-μL final volume of the amplification reaction included 1 μL of the first PCR reaction product, 0.2 μM primers (C1 and N1), 1.25 U of GoTaq DNA polymerase (5 U/μL), 1× GoTaq reaction buffer, and 0.2 mM deoxyribonucleotide triphosphate. The PCR amplification reaction was performed with the following calculated control protocol: a 30-second initial denaturation step at 94°C followed by 34 cycles of 15 seconds at 94°C, 30 seconds at 45°C, and 45 seconds at 72°C and a final extension of 10 minutes at 72°C. The PCR products were visualized by 2% agarose gel electrophoresis.
The real-time PCR targeting B1 gene was performed as described.21-24 Briefly, the primers used for amplifying the 126-bp B1 gene fragment were 5′-GGAGGACTGGCAACCTGGTGTCG-3′ (23 nt, TOX B1 F, forward primer) and 5′-TTGTTTCACCCGGACCGTTTAGCAG-3′ (25 nt, TOX B1 R, reverse primer). The hybridization probes were 5′-CGGAAATAGAAAGCCATGAGGCACTCC-FL (27 nt, TOX B1 FL, labeled at the 3′ end with fluorescein) and 5′-640-CGGAAATAGAAAGCCATGAGGCACTCC-3′ (27 nt, TOX B1 LC, labeled at the 5′ end with LC-Red 640, TIB Molbiol, Germany). The parasite quantification and melting curve analysis for each sample were performed with the 1.2 LightCycler real-time instrument with LightCycler software (version 4.0) according to the manufacturer's protocol (Roche). The 20-μL final volume of the PCR reaction included 5 μL of the purified patient DNA template or controls, 1× LightMix (TIB Molbiol), 1× FastStart mix (Roche), and 4 mM MgCl2. The PCR amplification reactions were performed with the following calculated control protocol: a 10-minute preincubation step at 95°C followed by 45 cycles of 10 seconds at 95°C, 5 seconds at 60°C, and 5 seconds at 72°C. As positive controls, T. gondii genomic DNA, serially 10-fold diluted and ranging from 5000 to 0.5 parasites per microliter (TIB Molbiol), and 1 negative control, prepared by the replacement of template DNA with distilled water, were used in each nested and real-time PCR run. Melting curve analysis was performed for the real-time PCR–positive samples after the quantification analysis with the following calculated protocol: a 20-second denaturation step at 95°C with a temperature transition rate of 20°C/second followed by a 20-second annealing step at 40°C with a temperature transition rate of 20°C/second and an extension step gradually increasing the temperature to 85°C with a temperature transition rate of 0.2°C/second.
Serological Analysis
Serum samples of liver recipients, donors, and controls were analyzed for anti-Toxoplasma IgM and IgG antibodies with the Sabin-Feldman dye test, EIA IgM, and ISAGA IgM.
The Sabin-Feldman dye test was performed as previously described.5, 25 Briefly, a suspension of 1 × 106 tachyzoites in 50% human complement was added to doubling dilutions of patient, donor, and control sera in sterile normal saline in flat-bottom microtiter plates (Serowel, United Kingdom). The endpoint titration was considered the dilution of serum required to elicit the killing of 50% of the tachyzoites. Human complement, obtained from the Blood Bank of Ege University Medical School, was tested for suitability and found negative for anti-Toxoplasma antibodies. The plates were incubated at 37°C for 1 hour, methylene blue was added, and the results were expressed in international units by a direct comparison with a toxoplasma reference (1000 units/mL of control serum supplied by National Institute for Biological Standards and Control, United Kingdom).

ISAGA IgM was performed according to the manufacturer's protocol (Biomerieux, France). Briefly, patient, donor, and control sera diluted in 1% bovine serum albumin [diluted in 1× phosphate-buffered saline (PBS)] were added in triplicate to the wells of the round-bottom microtiter plates coated with anti-human IgM monoclonal antibody, and they were incubated at 37°C for 2 hours. Then, the wells were washed 2 times with 1× PBS-T and 2 times with 1× PBS. T. gondii antigen, diluted 1:20 in bovine albumin borate saline buffer (pH 8.95) provided with the kit, was added to each well and incubated overnight at 37°C in a humidified environment. The results were interpreted by a direct comparison with controls provided with the kit.
RESULTS
PCR Analysis
Real-time and nested PCR detected toxoplasmosis in 3 liver transplant recipients from group 4 (patients 4, 13, and 17), and T. gondii DNA was not detected in groups 2, 3, and 5 (Table 1). Nested PCR was positive in patients 4 and 17. Real-time PCR was positive in patients 13 and 17. A blood sample of patient 4, collected 44 days after transplantation, was positive only by nested PCR (Fig. 1A). Blood samples of patient 17, collected 54 and 64 days after transplantation, were positive by both nested PCR and real-time PCR (Fig. 1A,B). Blood samples of patient 13, collected 27, 34, and 44 days after transplantation, were positive only by real-time PCR (Fig. 1B). Control samples were negative by both nested and real-time PCR (data not shown). During melting curve analysis of real-time PCR-positive samples, a shoulder at 62°C and a peak at 67.5°C (the T. gondii–specific melting point) were detected (Fig. 1C).
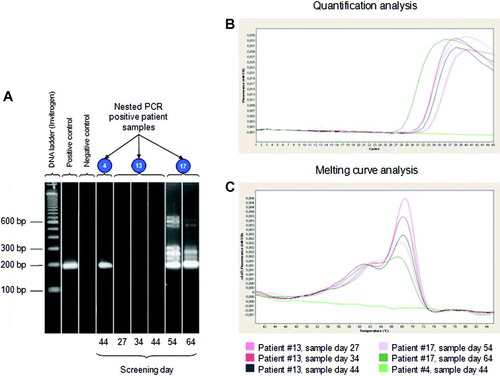
Results of nested and real-time PCR–positive liver recipient samples. Abbreviations: bp, base pairs; PCR, polymerase chain reaction.
A comparison of real-time PCR and nested PCR was undertaken in combination with a range of serological assays (the Sabin-Feldman dye test, EIA IgM, and ISAGA IgM). Ethylene diamine tetraacetic acid blood samples from 3 of the 30 recipients at risk from toxoplasma were found positive by PCR, but only 1 of these was found positive in both assays.
Serological Analysis
Serological analysis revealed alterations in the amount of antibodies only in patient serum samples from group 4, which were also PCR-positive (Table 1). A significant IgG level increase was detected by the Sabin-Feldman dye test only in patient 4 90 days after transplantation. A significant increase was not detected in the serum IgG level of patient 13 (Fig. 2A). A decrease in the serum IgG level of patient 17 taken 34 days after transplantation was measured (Table 2). IgM levels were below the cutoff value for patients 13 and 17 after transplantation by EIA (Table 2). Serum samples of patient 4, collected 34, 44, 54, and 64 days after the transplantation, were positive by EIA IgM (Fig. 2B). ISAGA IgM results were below the cutoff value after transplantation (Table 2). The patients who were negative by real-time and nested PCR showed no significant antibody increase during the screening.
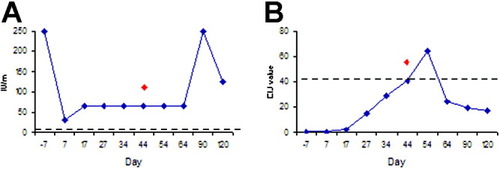
Correlation of the polymerase chain reaction and serological assay results of patient 4 during 4 months of screening: (A) serum immunoglobulin G levels (Sabin-Feldman dye test) and (B) serum immunoglobulin M levels (enzyme immunoassay immunoglobulin M). Nested polymerase chain reaction was positive at day 44 after transplantation (red diamonds). Dotted lines represent cutoff values.
| Patient Number* | Day −7 | Day 7 | Day 17 | Day 27 | Day 34 | Day 44 | Day 54 | Day 64 | Day 90 | Day 120 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 | SF dye test | 250 | 32 | 64 | 64 | 64 | 64 | 64 | 64 | 250 | 125 |
| EIA IgM | N | N | N | 15 | 29 | 41 | 64 | 24 | 19 | 17 | |
| IgM ISAGA | N | N | N | N | N | N | N | N | N | N | |
| Nested PCR | N | N | N | N | N | P | N | N | N | N | |
| Real-time PCR | N | N | N | N | N | N | N | N | N | N | |
| Symptoms | — | — | — | — | F | F, VD, H | F, VD | — | — | — | |
| Treatment | — | — | — | — | AB | CS, AB, G | TMP-SMX | TMP-SMX | — | — | |
| Prophylaxis | TMP-SMX | TMP-SMX | TMP-SMX | — | — | — | — | TMP-SMX | TMP-SMX | ||
| 13 | SF dye test | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| EIA IgM | N | N | N | N | N | N | N | N | N | N | |
| IgM ISAGA | N | N | N | N | N | N | N | N | N | N | |
| Nested PCR | N | N | N | N | N | N | N | N | N | N | |
| Real-time PCR | N | N | N | P | P | P | N | N | N | N | |
| Symptoms | — | — | — | F | F, VD, Na | F, VD | — | — | — | — | |
| Treatment | — | — | — | AB | AB | TMP-SMX | TMP-SMX | — | — | — | |
| Prophylaxis | TMP-SMX | TMP-SMX | TMP-SMX | — | — | — | — | TMP-SMX | TMP-SMX | TMP-SMX | |
| 17 | SF dye test | 64 | 64 | 64 | 64 | 32 | 32 | 32 | 16 | 32 | 32 |
| EIA IgM | N | N | N | N | N | N | N | N | N | N | |
| IgM ISAGA | N | N | N | N | N | N | N | N | N | N | |
| Nested PCR | N | N | N | N | N | N | P | P | N | N | |
| Real-time PCR | N | N | N | N | N | N | P | P | N | N | |
| Symptoms | — | — | — | — | — | — | F | F, H, Na | — | — | |
| Treatment | — | — | — | — | — | — | AB | AB, TMP-SMX | TMP-SMX | — | |
| Prophylaxis | TMP-SMX | TMP-SMX | TMP-SMX | TMP-SMX | TMP-SMX | TMP-SMX | — | — | — | TMP-SMX |
- NOTE: The SF dye test cutoff was 2 IU/mL; the EIA IgM EIU value of <25 and >40 was considered to be negative and positive, respectively; and the IgM ISAGA cutoff was 5. The transplantation date falls between day −7 and day 7.
- Abbreviations: AB, antibiotic treatment to prevent bacterial infection; CS, corticosteroid; EIA, enzyme immunoassay; F, fever; G, gentamicin ophthalmic drop; H, headache; IgM, immunoglobulin M; ISAGA, immunosorbent agglutination assay; N, negative; Na, nausea; P, positive; PCR, polymerase chain reaction; SF, Sabin-Feldman; TMP-SMX, trimethoprim-sulfamethoxazole; VD, visual disturbances.
- * The donors were seropositive (IgG+ and IgM−)
DISCUSSION
During profound immunosuppressive treatment to prevent organ rejection, toxoplasma can reactivate in transplant patients and may cause serious health problems with high morbidity and mortality.1, 5, 7, 10 The mortality and morbidity rates of toxoplasmosis in liver transplant patients are high because of the potential for late diagnosis and delayed treatment,8, 9 even though toxoplasmosis is reported to be less common in liver transplant patients.2, 6, 14, 28 Indeed, only 17 cases of toxoplasmosis have been reported in liver transplant recipients since 1972.2-6 In 2 previous studies, the incidence of toxoplasmosis in liver transplant recipients was 0.9% and 2.5%.6, 28 However, in another study, a 20% seroconversion rate was reported in a group of seronegative liver recipients with seropositive donors.5
Serological assays have been reported to be unreliable or insufficient after the initiation of intense immunosuppressive treatment in transplant patients because of the total antibody decrease due to leukoneutropenia.2, 10, 12-14, 29 Currently, the diagnosis of toxoplasmosis in transplant patients is commonly based on nested PCR and, more recently, real-time PCR because these have been reported to have high sensitivity and specificity.2, 9, 12, 15-19, 21-23 Thus, in the present study, a group of liver recipients under immunosuppressive treatment were screened for 4 months with real-time PCR, nested PCR, the Sabin-Feldman dye test, EIA IgM, and ISAGA IgM in order to compare the diagnostic value of these assays. We believe that this is the first time such a systematic comparison of this range of methods has been presented.
According to the results of initial serological screening (Table 1), transplant recipients in groups 3, 4, and, 5 were found to be seropositive (27/40, 67.5%) for toxoplasma infection and therefore at risk of reactivated infection. The serological status of the donors, which was ascertained in 38 of 40 cases, showed that 3 (7.9%) of the 38 recipients belonging to group 2 (D+/R−) were at risk of primary infection. In group 1 [10 (26.3%) of the 38 transplants], both the donor and recipient were seronegative, and this excluded toxoplasma as a risk during liver transplantation. Through the combination of the data from both PCR methods, the overall incidence of toxoplasmosis associated with liver transplantation was found to be 10% (3/30 cases). As primary infection was not detected (group 2) and all 3 cases were reactivations from group 4, the incidence of reactivated toxoplasmosis associated with liver transplantation was 11.1% (3/27; groups 3, 4, and, 5). Interestingly, among these 3 patients, 2 patients were positive by nested PCR or real-time PCR, but only 1 patient was positive by both PCR methods (Table 2). A similar discrepancy between nested and real-time PCR results has been reported previously.30-32
In primary toxoplasmosis or reactivation among liver recipients, total antibody levels have been reported to slightly increase or even decrease because of leukoneutropenia due to intense immunosuppressive treatment.2, 3, 5, 7, 10, 12, 19, 29, 33 In the present study, according to the results of the serological assays, IgG and IgM levels increased only in PCR-positive patient 4, IgG levels decreased in PCR-positive patient 17, and antibody levels remained unchanged in PCR-positive patient 13 (Table 2). In the previously reported 12 toxoplasmosis cases in liver recipients, IgG levels significantly increased in 6 patients, seroconversion occurred in 1 patient, and IgG levels remained constant in 5 patients. Among these cases, PCR and serology were performed together in 7 patients, in which PCR and serology were positive in 6 cases and PCR was positive in 1 seronegative patient.2-6 Thus, the apparent disagreement between serological findings and PCR methods may be explained by the reduction of antibody levels associated with intense immunosuppressive treatment.
Clinical manifestations of toxoplasmosis in immunosuppressed transplant recipients commonly present as fever, headache, nausea, lethargy, seizures, tremor, dyspnea and respiratory distress, hypoxemia, and visual disturbances due to brain (toxoplasmic encephalitis), eye (chorioretinitis), and lung (pneumonitis) involvement.1, 34 In the present study, positive PCR findings correlated with fever in 3 patients from group 4. In patient 4, visual disturbances and headache occurred, in patient 13, visual disturbances and nausea occurred, and in patient 17, headache and nausea occurred in addition to fever. Following the appearance of symptoms, toxoplasmosis was considered in the differential diagnosis by the clinicians, and laboratory findings did not identify any concomitant agent other than T. gondii. Interestingly anti-Toxoplasma prophylaxis had been withdrawn prior to these clinical changes in all of the PCR-positive patients, and following reinstatement of the treatment, the PCR findings reverted to negative and the symptoms resolved. There was no rejection episode during the course of toxoplasmosis (Table 2).
The results of the present study underline the value of PCR testing as an adjunct to serological screening in immunosuppressed patients when the clinician suspects active toxoplasmosis. Indeed, our findings suggest that PCR may be the most helpful single assay for clinicians in identifying active or reactivated toxoplasma infection. Furthermore, given that we observed evidence of active infection resulting in clinical sequelae in 10% of recipients when the recipient and/or donor was seropositive, we conclude that routine monitoring by PCR should be considered in this clinical group. We believe that this may be especially important to consider in the period immediately following cessation of anti-Toxoplasma prophylaxis to exclude reactivation and, where reactivation might occur, to permit more timely and effective treatment to prevent progression to the more severe symptoms that are otherwise typical in such cases.
Acknowledgements
The authors are pleased to acknowledge Dr. Metin Korkmaz for the evaluation of the serological tests and the team of the Toxoplasma Reference Unit at the Department of Microbiology of Singleton Hospital (Swansea, United Kingdom) for its help with various tests.




