Comparison of histidine-tryptophan-ketoglutarate solution and University of Wisconsin solution in extended criteria liver donors†
This article was presented at the World Transplant Congress, Boston, MA, 2006.
Abstract
Liver, pancreas, and kidney allografts preserved in histidine-tryptophan-ketoglutarate (HTK) and University of Wisconsin (UW) solutions have similar clinical outcomes. This study compares HTK and UW in a large number of standard criteria donor (SCD) and extended criteria donor (ECD) livers at a single center over 5 years. All adult, cadaveric liver and liver-kidney transplants performed between July 1, 2001 and June 30, 2006 were reviewed (n = 698). There were 435 livers (62%) categorized as ECD for severe physiologic stress and 70 (10%) because of old age. Recipient outcomes included perioperative death or graft loss and overall survival. Liver enzymes were analyzed for the first month post-transplant. Biliary complications were assessed through chart review. Overall, 371 donor livers were preserved in HTK (53%), and 327 were preserved in UW (47%). There were no statistically significant differences in any of the primary outcome measures comparing HTK and UW. The HTK group overall had a higher day 1 median aspartate aminotransferase and alanine aminotransferase, but the two groups were similar in function thereafter. HTK was superior to UW in protection against biliary complications. Kaplan-Meier graft survival curves failed to demonstrate a significant difference in SCD or ECD livers. In conclusion, HTK and UW are not clinically distinguishable in this large sample of liver transplants, although HTK may be protective against biliary complications when compared to UW. These findings persisted for both SCD and ECD livers. Given the lower cost per donor for HTK, this preservation solution may be preferable for general use. Liver Transpl 14:365–373, 2008. © 2008 AASLD.
An increasing number of organ procurement organizations (OPOs) and transplant centers have adopted histidine-tryptophan-ketoglutarate (HTK) preservation solution as their primary solid organ preservation solution. HTK has been shown to have clinical outcomes equivalent to those of University of Wisconsin (UW) solution while providing a significant cost savings.1 Specifically, previously published papers have demonstrated that HTK and UW have similar immediate graft function and graft and patient survival in kidney,2-4 pancreas,5-8 and liver transplantation.9, 10 No previous study has compared HTK and UW for extended criteria donors (ECDs) to determine if one solution is superior to the other in certain high-risk donor subgroups. This article presents the largest published prospective single-center comparison of HTK and UW in liver transplant patients and provides a subgroup comparison of a large volume of ECD liver recipients.
The compositions of HTK and UW preservation solutions and the history behind their development have been published previously.11 Briefly, HTK is a very low viscosity solution that is based on a buffer system (histidine) with two additional substrates (tryptophan and ketoglutarate). Initial descriptions of HTK usage involved flushing of the donor organs with 10-15 L of the solution, although our group has shown that infusion with 3-4 L results in equivalent clinical outcomes.6, 9 UW is a much more viscous solution that flushes at a slower rate and with a lower total volume. Preservation of the organs is based on three principles: (1) osmotic concentration maintained by metabolically inert substrates, (2) additional administration of the colloid carrier hydroxyethylstarch, and (3) addition of oxygen radical scavengers. UW is felt to provide organ tolerance to long cold ischemia times in a predictable manner. UW was first compared to HTK in a randomized fashion in liver transplantation over a decade ago.10 In 60 patients, the two solutions were found to have similar outcomes, which included initial nonfunction, initial liver function tests (LFTs), intensive care unit (ICU) stay, fresh frozen plasma use, and 30-month patient survival. Recent studies have included large case series from Europe12, 13 and North America,9 in which liver transplant outcomes have been equivalent for the two solutions. The solutions have also been found to be similar in living donor liver transplantation.14, 15
Our liver transplant program has increased its use of ECD livers between 2002 (60%) and 2006 (83%), and this has resulted in a significant decrease in waitlist size and median waitlist time for transplantation as well as decreased deaths on the waitlist.16 The routine use of ECD livers in transplantation has increased with the worsening shortage of donor organs. There is no consensus definition for extended criteria organs in liver transplantation. Factors thought to be associated with worse outcomes and increased risk of primary nonfunction and graft failure include increasing donor age, donation after cardiac death, increasing percent steatosis, elevated liver enzymes, severe hypernatremia, cold ischemia time over 12 hours, prolonged donor ICU stay with pressor use, and serology positive for hepatitis C virus (HCV), hepatitis B core antibody (HBcAb), or human T-lymphotropic virus (HTLV) 1/2. Use of these organs nationwide ranges from 10% to 20%. Recently, a donor risk index has been proposed with a regression model that suggests an increasing donor risk for increasing donor age, death from stroke or anoxia, African American race, donation after cardiac death, partial/split graft, decreasing donor height, increasing cold ischemia time, and regional or national sharing.17 Potential interaction between these extended donor criteria and choice of organ preservation solution has not been addressed in the literature.
Between July 1, 2001 and June 30, 2006, our center performed 698 adult, whole organ deceased donor liver transplants, 371 with HTK and 327 with UW preservation. This study compares HTK and UW for immediate and long-term transplant outcomes in this population, with an additional survival analysis within ECD subgroups. Our primary outcomes include perioperative death and graft loss and 3-month and 1-year graft and patient survival. Posttransplant liver function is assessed by comparison of liver enzymes at posttransplant days 1, 7, 14, and 30. Biliary complications are assessed with an extensive review of patient records and imaging studies.
Abbreviations
(Age)ECD, age extended criteria donor; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ECD, extended criteria donor; HBcAb, hepatitis B core antibody; HCV, hepatitis C virus; HTK, histidine-tryptophan-ketoglutarate; HTLV, human T-lymphotropic virus; ICU, intensive care unit; LFT, liver function test; MELD, Model for End-Stage Liver Disease; OPO, organ procurement organization; (P)ECD, physiologic extended criteria donor; SCD, standard criteria donor; UW, University of Wisconsin solution.
PATIENTS AND METHODS
Records for all adult liver transplants performed over the 5-year study period were reviewed (n = 698). The type of preservation solution and total volume infused for all locally procured and imported organs were obtained from our OPO records. Information regarding type of preservation solution was not routinely requested at the time of donor liver offer and had no bearing on the decision to use or to reject an organ. Cold and warm ischemia times for each organ were obtained from the United Network for Organ Sharing database. After May 1, 2003, the primary preservation solution for our OPO was changed from UW to HTK. The infused volumes of preservation solution and associated costs have been published previously by our group.9 Our mean infused volumes for HTK and UW are 3.8 and 3.2 L, respectively. Routine use of HTK corresponds with a cost savings of $422 per donor for the local OPO. The majority of the transplants (>90%) were performed with the piggyback hepatectomy technique. However, the transplants performed with a conventional approach occurred in the earlier period of this study when UW was the primary solution. This results in a much longer median warm ischemia time for UW when compared to HTK. An intraoperative prereperfusion portal flush was done routinely for both UW and HTK with 3 L of colloid solution (normal saline and albumin). Although this flush is not required with HTK according to the manufacturer, it was used for all transplants to maintain standardization of the operation and to wash out any excess potassium that had accumulated during the ischemic phase. A continuous intravenous aprotinin drip was routinely infused throughout the transplant. The median cold and warm ischemia times were 7 hours and 36 minutes, respectively. Overall median operative time was 270 minutes.
Posttransplant immunosuppression included induction with rabbit anti-thymocyte globulin (total: 6 mg/kg) with a rapid steroid taper. Maintenance immunosuppression was with tacrolimus monotherapy in most cases (goal maintenance level: 8 ng/DL). Our center currently delays all immunosuppression for 48 hours post-transplant so that the first dose of thymoglobulin and solumedrol is given on posttransplant day 2. There were 273 recipients who received a single dose of rituximab on postoperative day 3 as part of the induction protocol. Acute rejection in this cohort was rare (<5%). Rejection was treated with 1 to 3 doses of intravenous solumedrol (500 mg), depending on initial severity of rejection and clinical response to treatment. There were no events of graft loss due to rejection. All patients received cytomegalovirus and Pneumocystis carinii pneumonia prophylaxis therapy. The initial study of choice post-transplant for elevated liver enzymes at our center is Doppler ultrasound and endoscopic retrograde cholangiopancreatography, as indicated. These studies are followed by liver biopsy if no correctable pathology is identified. This protocol results in a large volume of endoscopic retrograde cholangiopancreatography studies in our post–liver transplant patients.
Liver donor demographic and clinical data were obtained from the original donor charts as recorded by the onsite OPO representative. An extensive literature review was undertaken prior to the data collection phase of this study to determine those donor factors that have been previously cited as potentially impacting liver transplant outcome. The three primary study groups for this analysis are listed in Table 1. Our ECD criteria have been published previously.16 However, it is unlikely that certain ECD criteria interact with the choice of preservation solution to impact transplant outcome (for example, reactive donor serologies and donor history of cancer). Therefore, this study compares three distinct groups: (1) standard criteria donors (SCDs), who do not meet any ECD criteria; (2) elderly donors {age extended criteria donors [(Age)ECDs]}, who are age 60 years or older; and (3) physiologic extended criteria donors [(P)ECDs], in which the allograft likely has significant perioperative physiologic stress. The criteria and their definitions are contained in Table 1.
| Number (%) | |
|---|---|
| TOTAL NUMBER | |
| (A) Standard criteria donors | 209 (30%) |
| (B) Donor age ≥ 60 years | 70 (10%) |
| (C) Physiologic extended criteria donors | 435 (62%) |
| Reperfusion biopsy steatosis ≥30% | 12 (2%) |
| Maximum donor serum Na+ ≥ 170 mmol/L | 86 (12%) |
| Maximum donor total bilirubin ≥ 2.0 | 82 (12%) |
| Maximum donor AST ≥ 500 | 56 (8%) |
| Maximum donor ALT ≥ 500 | 34 (5%) |
| Elevated donor LFTs (AST/ALT or bilirubin) | 136 (20%) |
| Donation after cardiac death | 28 (4%) |
| Donor liver cold ischemia time > 12 hours | 20 (3%) |
| Donor liver warm ischemia time > 60 minutes | 165 (24%) |
| 3 or more pressors simultaneously in donor | 82 (12%) |
| Donor ICU stay 6 days or more | 75 (11%) |
| Significant donor liver trauma (> Grade I injury) | 16 (2%) |
Graft and patient survival data were collected from the transplant database at our center. Patients are closely followed post-transplant by our center, which is the only liver transplant program in the state, but it is possible that some patients could have been unknowingly lost to follow-up. Patients and families were not individually contacted to confirm survival status. All recipients were listed for transplantation according to standard procedures and protocols established by the United Network for Organ Sharing. The mean Model for End-Stage Liver Disease (MELD) score at transplant was 18 (range: 6–48). MELD scores include point adjustments for patients with hepatocellular carcinoma. Patients receiving combined liver and kidney transplantation were included in the analysis, but those receiving other multiorgan transplants were excluded. For this cohort, there were no ABO mismatches. Donor liver steatosis was recorded as determined by pathology review of the reperfusion biopsy and included total steatosis (macrosteatosis and microsteatosis).
Transplant outcomes included intraoperative death, graft loss within 7 days of transplant, and 90-day and 1-year graft and patient survival. Initial graft function was analyzed with 1-, 7-, 14-, and 30-day aspartate aminotransferase (AST), alanine aminotransferase (ALT), and total bilirubin. All patients received a minimum of once daily labs while in-patient and twice weekly labs in the first month post-transplant. All of these labs were recorded in our transplant database and were extracted for this analysis. All occurrences of graft loss or patient death were included in the final analysis regardless of the etiology. There were no exclusions for intraoperative or perioperative mortality or graft loss or for non–liver-related deaths. In patients receiving a second transplant within 30 days of the first transplant, the second transplant was excluded from analysis, although the first transplant remained in the final results. Minimum follow-up time was 12 months, with median follow-up time of 40 months. This study was reviewed and approved by the Indiana University School of Medicine institutional review board.
Statistical analysis was conducted with commercially available software (SPSS version 14.0, 2004, SPSS, Inc.). Graft and patient survival outcomes were analyzed with the chi-square test and Fisher's exact test, as indicated. Kaplan-Meier survival curves with log rank testing were used to compare HTK and UW for graft survival. Differences in outcomes for UW and HTK within the study groups were considered significant for a P value < 0.05. Further comparison of HTK and UW for graft survival was performed within each of the ECD subgroups.
RESULTS
During the study period, 698 adults underwent whole organ, deceased donor liver or combined liver/kidney transplantation. The study groups were composed of SCDs [n = 209 (30%)], (Age)ECDs [n = 70 (10%)], and (P)ECDs [n = 435 (62%); Table 1]. Intergroup demographics were similar, with the following exceptions. Within the SCD group, a greater percentage of retransplants had livers preserved in HTK (10.8% versus 3.1% in UW, P = 0.03). All three study groups differed significantly in cold and warm ischemia times, with UW-preserved livers having longer cold and warm ischemia times in both the ECD and SCD study groups (P < 0.001 for HTK versus UW in all groups; Table 2). The difference in ischemia times resulted from improved efficiency in organ procurement, transport, and transplant in the latter years of the study when HTK was the primary preservation solution.
| (A) SC (n = 209) | (B) (Age) EC (n = 70) | (C) (P)EC (n = 435) | ||||
|---|---|---|---|---|---|---|
| HTK | UW | HTK | UW | HTK | UW | |
| TOTAL | n = 111 (53%) | n = 98 (47%) | 40 (57%) | 30 (43%) | 204 (47%) | 231 (53%) |
| Recipient gender | ||||||
| Male | 70 (63%) | 68 (69%) | 26 (65%) | 18 (60%) | 130 (64%) | 155 (67%) |
| Recipient race | ||||||
| White | 98 (88%) | 91 (93%) | 37 (93%) | 28 (93%) | 183 (90%) | 207 (90%) |
| Black | 9 (8%) | 4 (4%) | 2 (5%) | 0 (0%) | 12 (6%) | 13 (5%) |
| Other | 4 (4%) | 3 (3%) | 1 (2%) | 2 (7%) | 9 (4%) | 11 (5%) |
| Recipient age (years) | ||||||
| Median, range | 51, 21 to 76 | 49, 18 to 72 | 55, 30 to 69 | 55, 41 to 71 | 53, 22 to 71 | 51, 18 to 71 |
| Recipient BMI | ||||||
| Median, range | 28, 16 to 39 | 27, 18 to 40 | 29, 17 to 40 | 30, 18 to 38 | 28, 16 to 40 | 28, 18 to 42 |
| Recipient/donor ABO | ||||||
| A | 50 (45%) | 39 (40%) | 9 (23%) | 13 (43%) | 95 (47%) | 89 (39%) |
| B | 9 (8%) | 7 (7%) | 6 (15%) | 3 (10%) | 23 (11%) | 26 (11%) |
| AB | 0 (0%) | 1 (1%) | 1 (2%) | 1 (3%) | 3 (1%) | 7 (3%) |
| O | 52 (47%) | 51 (52%) | 24 (60%) | 13 (43%) | 83 (41%) | 109 (47%) |
| Recipient MELD at transplant | ||||||
| Median, range | 18, 7 to 43 | 17, 6 to 40 | 16, 10 to 24 | 15, 10 to 30 | 16, 7 to 48 | 16, 6 to 41 |
| Retransplant | 12 (11%) | 3 (3%)** | 0 (0%) | 1 (3%) | 4 (2%) | 9 (4%) |
| Donor gender | ||||||
| Male | 62 (56%) | 59 (60%) | 15 (38%) | 10 (33%) | 115 (56%) | 133 (58%) |
| Donor age (years) | ||||||
| Median, range | 38, 9 to 59 | 38, 10 to 59 | 64, 60 to 81 | 65, 60 to 75 | 39, 6 to 76 | 42, 8 to 74 |
| Donor BMI | ||||||
| Median, range | 25, 14 to 33 | 25, 16 to 35 | 26, 18 to 41 | 26, 18 to 45 | 26, 13 to 50 | 26, 14 to 47 |
| Donor race | ||||||
| White | 94 (85%) | 88 (90%) | 36 (90%) | 25 (84%) | 171 (84%) | 190 (82%) |
| Black | 14 (13%) | 7 (7%) | 3 (8%) | 4 (13%) | 20 (10%) | 31 (14%) |
| Other | 3 (3.3%) | 3 (3%) | 1 (2%) | 1 (3%) | 13 (6%) | 10 (4%) |
| Total cold ischemia time (hours) | ||||||
| Median, range | 6, 3 to 12 | 8, 3 to 12** | 7, 4 to 10 | 8, 5 to 11** | 7, 3 to 16 | 8, 3 to 20** |
| Total warm ischemia time (minutes) | ||||||
| Median, range | 30, 16 to 138 | 59, 20 to 125** | 28, 15 to 142 | 59, 21 to 120** | 31, 13 to 142 | 62, 19 to 203** |
- *Categorical variables compared using chi-square test. Continuous variables compared using Mann-Whitney test.
- ** P -value < 0.05 for comparison of HTK and UW within subgroup.
HTK and UW were found to have no statistically significant difference in posttransplant graft and patient survival, risk of intraoperative death, and graft failure in the first 7 days post-transplant. These findings were consistent within the three study groups (Table 3). Evaluation of early graft loss and graft loss within the first year post-transplant for (P)ECD livers is displayed in Table 4. A review of the results in this table does not indicate a significant difference between HTK and UW for any (P)ECD category. In the important areas of cold ischemia time and donor age, the two solutions are clinically equivalent. The Kaplan-Meier liver allograft survival curves are shown in Figs. 1-3. The HTK and UW curves are nearly indistinguishable up to 1-year post-transplant.
| (A) SCD (n = 209) | (B) (Age)ECD (n = 70) | (C) (P)ECD (n = 435) | ||||
|---|---|---|---|---|---|---|
| HTK | UW | HTK | UW | HTK | UW | |
| Intraoperative death | 0/111 (0%) | 2/98 (2%) | 0/40 (0%) | 1/30 (3%) | 4/204 (2%) | 4/231 (2%) |
| Graft loss within 7 days of transplant | 3/111 (3%) | 5/98 (5%) | 1/40 (2%) | 3/30 (10%) | 12/204 (6%) | 13/231 (6%) |
| 3-month survival | ||||||
| Graft | 101/111 (91%) | 88/98 (90%) | 33/40 (82%) | 24/30 (80%) | 182/204 (89%) | 204/231 (88%) |
| Patient | 102/111 (92%) | 90/98 (92%) | 33/40 (82%) | 24/30 (80%) | 185/204 (91%) | 207/231 (90%) |
| 1-year survival | ||||||
| Graft | 96/111 (86%) | 82/98 (84%) | 30/40 (75%) | 21/30 (70%) | 171/204 (84%) | 191/231 (83%) |
| Patient | 96/110 (87%) | 86/98 (88%) | 30/40 (75%) | 22/30 (73%) | 174/204 (85%) | 195/231 (84%) |
- HTK and UW are compared within each subgroup using the chi-square or Fisher's Exact Test, as appropriate.
- None of the statistical tests resulted in a p-value <0.05 for comparison of HTK and UW.
| Graft Loss Within 7 Days of Transplant | Graft Survival 3-Months | Graft Survival 1-Year | ||||
|---|---|---|---|---|---|---|
| HTK | UW | HTK | UW | HTK | UW | |
| All physiologic extended criteria donors | 12/204 (6%) | 13/231 (6%) | 182/204 (89%) | 204/231 (88%) | 171/204 (84%) | 191/231 (83%) |
| Reperfusion biopsy steatosis ≥ 30% | 0/7 (0%) | 1/5 (20%) | 6/7 (86%) | 4/5 (80%) | 6/7 (86%) | 3/5 (60%) |
| Maximum donor serum Na+ ≥ 170 mmol/L | 3/46 (6.5%) | 1/40 (2.5%) | 41/46 (89%) | 35/40 (88%) | 40/46 (87%) | 35/40 (88%) |
| Elevated donor liver function tests | 1/63 (1.6%) | 3/73 (4.1%) | 58/63 (92%) | 64/73 (88%) | 54/63 (86%) | 59/73 (81%) |
| Donation after cardiac death | 1/21 (5.0%) | 0/8 (0%) | 19/21 (90%) | 7/8 (88%) | 15/21 (71%) | 7/8 (88%) |
| Cold ischemia time > 12 hours | 1/6 (16.7%) | 2/14 (14.3%) | 4/6 (67%) | 11/14 (79%) | 4/6 (67%) | 11/14 (79%) |
| Warm ischemia time > 60 minutes | 2/35 (6%) | 8/130 (6%) | 32/35 (91%) | 114/130 (88%) | 28/35 (80%) | 106/130 (82%) |
| 3 or more pressors simultaneously in donor | 2/54 (3.7%) | 0/28 (0%) | 51/54 (94%) | 27/28 (96%) | 49/54 (91%) | 22/28 (79%) |
| Donor ICU stay greater than 5 days | 5/44 (11.4%) | 0/31 (0%) | 37/44 (84%) | 29/31 (94%) | 34/44 (77%) | 28/31 (90%) |
| Donor liver trauma > Grade 1 | 0/4 (0%) | 0/12 (0%) | 4/4 (100%) | 10/12 (83%) | 4/4 (100%) | 9/12 (75%) |
- * HTK and UW are compared within each subgroup using either the chi-square or Fisher's Exact Test, as appropriate. None of the statistical tests resulted in a p-value <0.05 for comparison of HTK and UW.
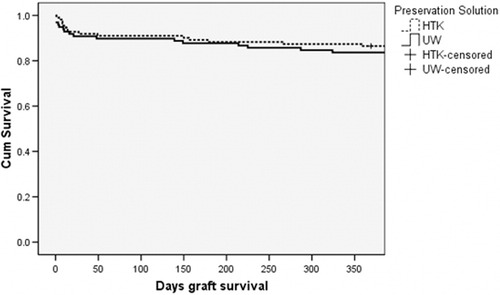
Kaplan-Meier graft survival for donor liver allografts from SCDs preserved in HTK (n = 111) or UW (n = 98) preservation solution (log rank P = 0.14).
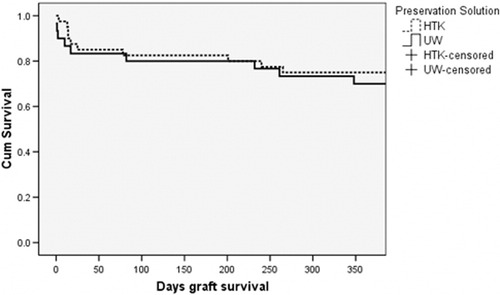
Kaplan-Meier graft survival for donor liver allografts age 60 years and older preserved in HTK (n = 40) or UW (n = 30) preservation solution (log rank P = 0.13).
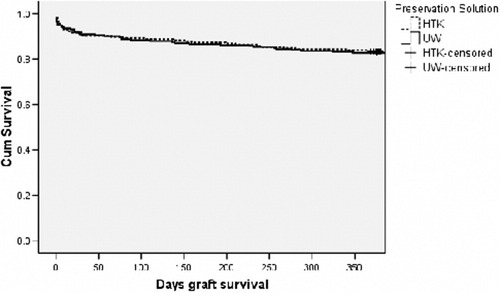
Kaplan-Meier graft survival for donor liver allografts from (P)ECDs preserved in HTK (n = 204) or UW (n = 231) preservation solution (log rank P = 0.19).
Initial liver function enzymes were measured and graphed at 1, 7, 14, and 30 days post-transplant (Figs. 4-6). Livers preserved with HTK have a higher initial AST and ALT in SCD and (P)ECD livers, whereas the UW-preserved livers have a higher AST and ALT in the old donor livers. In all groups, these differences disappear by postoperative day 7. The serum total bilirubin for the HTK and UW groups were similar throughout the first month and were equivalent by day 30. The older donor livers had a persistently higher total bilirubin level when compared to the SCD and (P)ECD groups (Fig. 7). Posttransplant biliary complications are listed in Table 5. HTK is superior to UW in every category measured, including any need for biliary evaluation and the presence of bile duct stones or sludge.
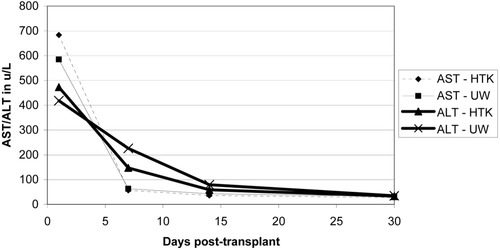
Liver enzymes in the first 30 days post-transplant for SCDs: comparing HTK (n = 111) and UW (n = 98) preservation solutions.
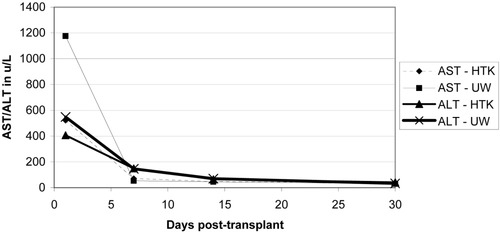
Liver enzymes in the first 30 days post-transplant for older donor livers (age 60 years and older): comparing HTK (n = 40) and UW (n = 30) solutions.
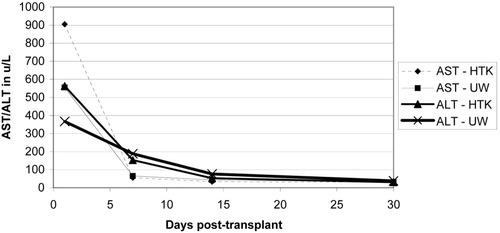
Liver enzymes in the first 30 days post-transplant for (P)ECDs: comparing HTK (n = 204) and UW (n = 231) solutions.
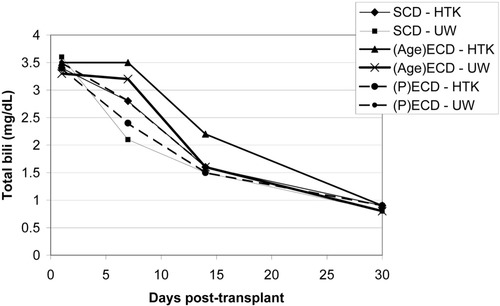
Serum total bilirubin in the first 30 days post-transplant for SCDs (n = 209), (Age)ECDs (n = 70), and (P)ECDs (n = 435).
| Overall | HTK | UW | P Value† | |
|---|---|---|---|---|
| Overall | 698* | 371* | 327* | |
| Need for biliary imaging | 384/693 (55.4%) | 188/367 (51.2%) | 196/326 (60.1%) | 0.01 |
| Choledochocholedochostomy (n = 593) | 333/593 (56.2%) | 163/316 (51.6%) | 170/277 (61.4%) | 0.02 |
| Roux-y hepaticojejunostomy (n = 100) | 51/100 (51.0%) | 25/51 (49.0%) | 26/49 (53.1%) | NS |
| Biliary leak | 22/693 (3.2%) | 9/367 (2.5%) | 13/326 (4.0%) | NS |
| Diffuse intrahepatic stricturing | 26/693 (3.8%) | 9/367 (2.5%) | 17/326 (5.2%) | NS |
| Presence of choledocholithiasis | 115/693 (16.6%) | 48/367 (13.1%) | 67/326 (20.6%) | 0.06 |
| Presence of bile duct gravel (sludge) | 51/693 (7.4%) | 14/367 (3.8%) | 37/326 (11.3%) | 0.001 |
- * Five patients died intraoperatively and had no biliary reconstruction (4 HTK and 1 UW).
- † NS indicates P > 0.10.
DISCUSSION
HTK has been adopted by many transplant centers for routine use in procurement of liver, kidney, and pancreas allografts. We have previously reported results from our center comparing HTK and UW as the primary preservation solution in liver,9 kidney,2, 3 and pancreas5, 6 transplantation. This study is the only report comparing UW and HTK in a single center using a large number of extended criteria liver donors. From these data, it appears that the two solutions are indistinguishable in clinical transplant outcomes for both the ECD and SCD groups, including perioperative, 3-month, and 1-year graft and patient survival. The 1-month initial LFTs were clinically similar. This study did not demonstrate clear superiority of either HTK or UW within the SCD, (Age)ECD, or (P)ECD subgroups. Further analysis within the (P)ECD subgroups also failed to demonstrate a statistically significant difference.
Eghtesad and colleagues18 recently reviewed the use of HTK and UW in liver transplantation. Comparing HTK and UW in liver transplantation fails to identify any convincing evidence of superiority of one solution over the other. These authors list several potential benefits in using HTK. HTK has a much lower viscosity that may result in better penetration of the microcirculation for a better flush. This may lead to a lower rate of biliary complications and may provide a more thorough flush in livers procured with donation after cardiac death. This article also cites the low potassium content of HTK, which obviates the need for an in situ flush prior to revascularization. Our center has chosen to continue in situ flushing to standardize the operation, although this adds 3 minutes to our warm ischemia time. We have previously demonstrated an elevation in postoperative day 1 aminotransferases, but this difference disappears by postoperative day 7. There have been reports of an increased rate of late biliary complications in UW-preserved livers for both living donors and cadaveric organs.13, 14 Results from our center support these previous findings and suggest that HTK is protective against biliary complications when compared to UW.
We have previously published a comparison of HTK and UW for all liver transplants at our center (combined SCD and ECD donors).9 The incidence of primary nonfunction was 1%, with no difference based on preservation solution. The present subgroup analysis of ECD donors also demonstrates a low incidence of primary nonfunction, with HTK and UW being statistically similar. There is no difference in the rate of vascular thrombosis between the two groups. Additionally, there was no demonstrated difference in on-table deaths that may be a concern with the high potassium load at reperfusion associated with UW preservation.
This study and others have suggested that UW may perform better in donor livers with prolonged cold ischemia time. Only 20 of 698 donor livers in this population of donor livers had a cold ischemia time of 12 hours or greater, and this limits the conclusions that can be drawn. Our center strives to minimize cold ischemia time in all livers. Our median cold ischemia time over 698 liver transplants is 7 hours, with a median warm ischemia time of 36 minutes. These short times likely ameliorate the impact of preservation solution choice on outcome. This may explain, in part, the equivalence seen in the solutions. UW may perform better for prolonged cold ischemia time, but this cannot be determined from our data.
In conclusion, this study is the only published report comparing HTK and UW in routine use in the subset of ECD livers at a large-volume center. Results of this analysis suggest that graft function and patient and graft survival are equivalent between UW-preserved and HTK-preserved ECD livers within short cold and warm ischemia times. Either UW or HTK may be superior in certain clinical situations, such as prolonged cold or warm ischemia time, but further study would be required to delineate the impact of preservation solution on each of these factors individually. Certainly, HTK appears to have an advantage related to protection against biliary complications. This is likely secondary to an improved flush of the biliary microcirculation achieved by the less viscous HTK. A review of the available literature indicates that HTK and UW result in equivalent clinical outcomes for overall use in liver transplantation and for use in partial liver graft transplantation. This article now demonstrates equivalence of these two solutions for liver transplants from a large population of ECDs.




