Donor-transmitted malignancy confirmed by quantitative fluorescence polymerase chain reaction genotype analysis: A rare indication for liver retransplantation
The incidence of donor-derived malignancy is thought to be of the order of 0.02%–0.2%.1, 2 There have been reports of transmission from donor to recipient of a variety of malignancies, including malignant melanoma, choriocarcinoma, neuroendocrine tumors, and adenocarcinomas of the lung, prostate, and pancreas.3-9
Identifying the origin of a malignancy post transplant as being either donor-derived or recipient-derived has important clinical consequences, including withdrawal of immunosuppression and retransplantation. It also has implications for other recipients of donor organs. In sex-mismatched allografts, this distinction can be made with chromosomal analysis.5, 6 More recently, microsatellite analysis has allowed the detection of tumor lineage of both donor7, 10-14 and recipient origin.15, 16
We present a case of a liver transplant recipient found to have adenocarcinoma of donor origin and subsequently managed by retransplantation.
CASE REPORT
The patient was a 59-year-old Caucasian male, with a history of type II diabetes mellitus and hypertension, who underwent in August 2003 orthotopic liver transplantation secondary to hepatitis C–induced end-stage liver disease. The donor was known to be a 68-year-old fit and active male who died suddenly of a subarachnoid hemorrhage. Predonation screening questionnaires revealed no symptoms suggestive of malignancy, and a post mortem examination was not held. Two kidneys, corneas, and a heart valve have been transplanted from the same donation.
A routine posttransplant abdominal ultrasound scan in September 2004 identified 3 lesions within the liver suspicious for metastatic disease. Biopsy of the lesions confirmed adenocarcinoma of likely colorectal origin. Further investigations, including endoscopy, 2 colonoscopies and biopsy, chest/abdomen computed tomography, a bone scan, and a positron emission tomography scan, revealed no evidence of a primary carcinoma.
Microsatellite genotype analysis by quantitative fluorescence polymerase chain reaction (QF-PCR) of donor tissue and recipient DNA and tumor tissue revealed the adenocarcinoma to be of donor origin; as a result, the recipient was listed for retransplantation, and a second cadaveric liver transplant was performed in June 2005. The first allograft had 3 confirmed large metastatic deposits measuring 6.0, 5.2, and 4.2 cm, respectively. Each adenocarcinomatous deposit had a periphery of largely viable irregular glands, mingled with mucinous necrotic and inflammatory debris, that was underlain by largely necrotic and fibrotic tissue. The gland epithelium was for the most part columnar, exhibiting moderate anisokaryosis and frequently containing mitotic figures, consistent with origin from a large bowel primary site in the donor.
Following retransplantation, the patient developed significant recurrence of hepatitis C–related liver disease in the transplant. He also had ongoing medical problems with chronic renal impairment secondary to his diabetes and tacrolimus therapy and as such was not considered medically fit enough for adjunctive chemotherapy. As of June 2007, he remains tumor-free. All other recipients from the donor remain well.
Abbreviations
PCR, polymerase chain reaction; QF-PCR, quantitative fluorescence polymerase chain reaction.
MATERIALS AND METHODS
DNA was extracted from a section of normal donor liver tissue mounted in paraffin and a hematoxylin-eosin section of metastatic tumor tissue with proteinase K lysis. Germline DNA from the recipient was extracted from peripheral blood lymphocytes. All 3 DNA samples were independently polymerase chain reaction (PCR)–amplified by single-tube multiplex PCR assay with a QF-PCR approach.17 This assay requires just a small amount of DNA and amplifies 13 highly polymorphic markers on chromosomes 13, 18, and 21. The genotypes were analyzed and compared on a 3100 genetic analyzer with Genotype software (Applied Biosystems, Foster City, CA).
RESULTS
Tumor Characteristics
The results from 12 of the 13 tested markers were of sufficient quality for genotype analysis. Of these, 3 markers exhibited 4 different alleles in both the normal donor liver DNA and the metastatic tumor DNA (Fig. 1, D18S386). Two of the 4 alleles were the same as the recipient germline genotype. The additional 2 alleles therefore likely originated from the donor tissue and represent the donor genotype. The results from all 3 markers are consistent with a predominant donor genotype and a minor recipient genotype. Eight markers were partially informative and were consistent with this interpretation (Fig. 1, D13S634 and D21S1435). Together, these results are also consistent with the donor genotype being present at a higher level in the metastatic tumor tissue than in the normal liver DNA. The final marker exhibited the same genotype in all tissues tested and was therefore uninformative. In summary, genotype analysis is consistent with the metastatic adenocarcinoma diagnosed in the liver recipient, 13 months after transplantation, originating from the donor and not the recipient. The recipient genotype present in the normal liver and tumor samples is presumably due to the venous blood supply to these tissues.
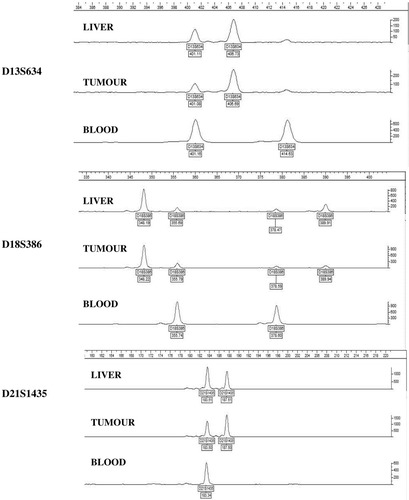
DNA from the recipient blood, liver and tumor tissue were PCR amplified for polymorphic markers on chromosomes 13, 18 and 21 by single-tube multiplex PCR assay using a QF-PCR approach. The results from the three markers are consistent with a predominant donor genotype and a minor recipient genotype.
DISCUSSION
Since the early days of transplantation, the risk of transmission of malignancy from donor to recipient has been recognized.18 In 2002, Birkeland and Storm1 attempted to quantify this risk by performing the first population-based analysis of unrecognized malignancies in cadaveric and living related donors. They estimated the risk of having a donor with an undetected malignancy to be 8 in 626 (1.3%), with a risk of 1 in 626 (0.2%) of donor-to-recipient transmission of cancer. Smaller studies have estimated that up to 5% of donors who appeared low-risk on traditional screening questionnaires had occult malignancy.19
Here we present the case of an adenocarcinoma of donor origin identified within an hepatic allograft by comparative analysis of recipient and donor DNA polymorphisms. The donor had no risk factors for occult carcinoma identified on routine questionnaire screening. Of note, recipients of kidneys, corneas, and a heart valve from the same donor remain disease-free after 45 months.
This case report represents one of a growing number of reports diagnosing donor-derived malignancy after the identification of a tumor in the recipient. What once proposed a diagnostic challenge has been rendered increasingly simple with modern molecular genetic techniques. This allows early and accurate diagnosis of the origin of malignant disease, which is essential for optimizing the management of the patient and other recipients.
These cases raise a number of significant issues. First, the transmission of cancer from a donor will always beg the question as to whether current donor screening protocols are sufficient. Morris-Stiff et al.20 reported transmission of multiple melanoma from a donor to multiple recipients. They argue that it is necessary for all donors to undergo post mortem examination to exclude metastatic disease. This would have a major impact in our current climate of critical organ shortage if it were implemented and have repercussions for out-of-hours pathology services. Furthermore, others have shown that recipients of organs from donors found at post mortem to have occult malignancy may remain disease-free long term and managed under close surveillance.21 Conversely, despite emergency retransplantation of such organs after discovery of malignant disease, death from metastatic donor-derived disease has been reported.11 The risk-benefit ratio of compulsory post mortem may not support its implementation.
The management of patients diagnosed with donor-derived malignancy also remains a matter for debate. This case, along with other similar cases,2, 5, 10, 11 gives some evidence that emergency retransplantation in hepatic grafts appears to be curative. The role for radioablation, immunosuppression, and chemotherapy is less clear-cut.
The management of tumor-free recipients from donors known to have transmitted a malignancy is uncertain. The risk to these individuals will depend on the histological grade and metastatic potential of the neoplasm involved. In this instance, colorectal adenocarcinoma metastases are common to liver tissue but not to heart valves or corneal tissue. There was no evidence of direct colorectal cancer spread to the kidneys at organ retrieval. No additional screening program has been put in place for these recipients. Analysis of data from sources such as the United Network for Organ Sharing Transplant Tumour Registry22 may aid in further evaluating this risk and allowing individual management plans to be made.
In summary, we have presented a case of an adenocarcinoma of donor origin diagnosed in a hepatic transplant recipient. This case once again opens the debate on whether more stringent examination of potential donors is necessary and offers further support for the role of emergency retransplantation in such patients and the need for close follow-up of other recipients of organs from the same donor.
Acknowledgements
The authors thank Helen Mandefield, the organ donation service manager of the South Thames Transplant Coordination Service.




