Incidence of severe ventricular arrhythmias during pulmonary artery catheterization in liver allograft recipients
Abstract
Liver allograft recipients may develop a hyperdynamic circulation and cardiac electrophysiologic abnormalities. The incidence of severe ventricular arrhythmias in liver allograft recipients during pulmonary artery (PA) catheterization was determined. One hundred five liver allograft recipients were studied prospectively; 5 of the patients with preexisting valvular heart disease, ischemic heart disease, or arrhythmias were excluded. Severe ventricular arrhythmia, defined as 3 or more consecutive ventricular premature beats occurring at a rate of >100 per minute, was observed in 37.0% of the patients during insertion of the catheter and in 25.0% of the patients during removal of the catheter. Two patients developed ventricular tachycardia, and 2 developed ventricular fibrillation; the arrhythmias in these 4 patients did not respond to appropriate pharmacological treatment but resolved promptly after removal of the PA catheter. The catheter transit time from the right ventricle to the pulmonary capillary wedge position was longer in patients with severe ventricular arrhythmia than in those without this arrhythmia (91.6 ± 103.6 s versus 53.3 ± 18.4 s, P < 0.05). In conclusion, patients undergoing liver transplantation have a high risk of developing a ventricular arrhythmia during PA catheterization. Liver Transpl 13:1451–1454. 2007. © 2007 AASLD.
Liver transplantation is commonly associated with the administration of large volumes of fluid and massive hemorrhaging; predisposing factors include abnormalities of blood coagulation, the presence of numerous collateral vessels, and prolonged periods of surgery. Patients with end-stage chronic liver disease have a hyperdynamic circulation, features of which include increased cardiac output, tachycardia, and decreased systemic vascular resistance (SVR). These changes are associated with extensive arteriovenous communications and release of systemic vasodilators, such as glucagon, nitric oxide, ferritin, and vasoactive intestinal polypeptide.1 Severe pulmonary hypertension or intrapulmonary arteriovenous shunts also occur in such patients.2 Severe pulmonary hypertension is a contraindication to liver transplantation. Assessments of ventricular function are required throughout this procedure. Accordingly, in most centers, a pulmonary artery (PA) catheter is inserted routinely to evaluate cardiac function. The development of arrhythmias during the insertion of a PA catheter in patients undergoing cardiovascular surgery has been reported3, 4 (Fig. 1). However, the incidence of arrhythmias and hemodynamic changes in relation to the placement of a PA catheter in liver allograft recipients is unknown. Our experience with such patients suggests that the development of an arrhythmia commonly occurs during the insertion and removal of the catheter. The aim of this study was to examine prospectively arrhythmias and hemodynamic changes associated with PA catheterization in liver allograft recipients and to investigate possible factors responsible for these complications.
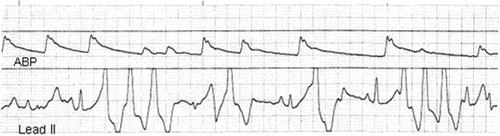
Ventricular tachycardia induced by the insertion of a pulmonary artery catheter.
Abbreviations
ASA, American Society of Anesthesiologists; CVP, central venous pressure; ECG, electrocardiogram; PA, pulmonary artery; QTC, rate-corrected QT interval; SVR, systemic vascular resistance.
PATIENTS AND METHODS
This study was approved by the local institutional review board on human studies; informed consent was waived. A total of 105 patients undergoing liver transplantation were studied prospectively. Five patients with preexisting valvular heart disease, ischemic heart disease, or arrhythmias were excluded. After the induction of anesthesia, the right internal jugular vein was cannulated, and an 8.5-French introducer sheath for the PA catheter was inserted with the percutaneous Seldinger technique while patients were in the Trendelenburg position. No intravenous lidocaine was given prophylactically. A 7.5-French balloon-tipped flow-directed PA catheter (model 774HF75, Edwards Lifesciences LLC, Irvine, CA) was advanced through the introducer while patients were positioned with their head held up at an angle of 15-20° with a right lateral tilt. The catheterization was undertaken by a third-year anesthesia resident who had been assigned to the procedure; all residents had prior education and experience of PA catheterization. All the procedures were directly supervised by either of the 2 attending anesthesiologists (M.S.G. and G.S.K.). The catheter was advanced to the 20-cm mark, and the balloon was inflated with 1.5 mL of air. The catheter was advanced through the right ventricle into the PA and subsequently wedged in a peripheral PA under the guidance of a pressure waveform (1.5 cm/s). Electrocardiogram (ECG) tracings (leads II and V5), systemic arterial pressure, and the pressure at the PA port were recorded as catheters were advanced from the right atrium to the pulmonary capillary wedge position during insertion and again during removal of the PA catheters. Two anesthesiologists, who were blinded to the study, independently reviewed the ECG and pressure recordings and classified arrhythmias as absent, benign, or severe. Benign arrhythmias included premature atrial contractions, transient right bundle branch block, isolated premature ventricular contractions, couplets, or bigeminy at a rate of <100 beats per minute. Severe ventricular arrhythmia was defined as 3 or more consecutive ventricular premature beats at a rate of >100 per minute.5 The observed ECG tracings were classified according to the severest type of arrhythmia that occurred. The time taken to advance the PA catheter from the right ventricle to the wedge position was recorded. Arterial blood gas analysis, blood cell counts, and serum levels of electrolytes were recorded. A 12-lead preoperative ECG was recorded for a computerized assessment of the QRS complex and mean QT interval. The rate-corrected QT interval (QTc) was calculated as follows: QTc = QT interval (seconds)/square root of RR interval (seconds).6 According to current criteria, a QTc > 440 milliseconds is considered to be prolonged.
Data were expressed as means ± standard deviation and were analyzed, where appropriate, with the Student t test or the chi-square test. A P value < 0.05 was considered to be significant.
RESULTS
All 105 patients were catheterized successfully. Five patients were excluded from the study because of underlying valvular or ischemic heart disease or arrhythmias. Demographic information is given in Table 1. Arrhythmias occurred in 70% of patients during insertion of the PA catheter. Thirty-three patients (33%) developed a benign arrhythmia, and 37 patients (37%) developed a severe ventricular arrhythmia. All the arrhythmias were catheter-induced and self-limiting; they subsided once the catheter had been advanced into the PA or had been withdrawn into the superior vena cava. During surgery, 4 patients with normal serum electrolyte levels developed severe ventricular arrhythmias: ventricular tachycardia associated with hemodynamic instability in 2 and ventricular fibrillation in the other 2. These arrhythmias were not corrected by appropriate pharmacological treatment, such as epinephrine or lidocaine, or, in one case, by defibrillation; however, they subsided promptly after complete removal of the PA catheter. PA catheters were removed from the remaining 96 patients after surgery. In these patients, arrhythmias were observed during removal in 48 patients (50%); these were benign in 25% (24/96) and severe in 25% (24/96). The incidence of arrhythmias when the tip of the catheter was positioned in the right atrium, right ventricle, and PA was 5.6%, 44.4%, and 50% during insertion, respectively, and 16.7%, 75%, and 8.3% during removal, respectively.
| Age (years) | 49.8 ± 8.8 |
| Sex (male/female) | 76/24 |
| Weight (kg) | 67.5 ± 10.5 |
| Height (cm) | 166.0 ± 8.4 |
| Child-Pugh classification (A:B:C) | 4:24:72 |
| ASA classification (1:2:3:4) | 0:13:78:9 |
| Etiology (alcohol:hepatitis B virus:hepatitis C virus:primary biliary cirrhosis:others) | 13:71:7:6:3 |
| QTc (milliseconds) | 439.1 ± 25.8 |
| Hypertension | 9 (9.0%) |
| Diabetes mellitus | 28 (28.0%) |
- NOTE: Values are reported as the mean ± standard deviation or number of patients (%).
- Abbreviations: ASA, American Society of Anesthesiologists; QTc, rate-corrected QT interval on lead II of the electrocardiogram [measured QT interval (seconds)/square root of RR interval (seconds)].
Patients with severe ventricular arrhythmia had a longer transit time of the catheter from the right ventricle to the pulmonary capillary wedge position (91.6 ± 103.6 s) than those without severe ventricular arrhythmia (53.3 ± 18.4 s, P < 0.05). The mean arterial pressures for the group with severe ventricular arrhythmia and for that without severe ventricular arrhythmia (measured immediately before catheter insertion) were significantly different during both insertion and removal of the catheter (P < 0.05). The average calculated SVR was 724.6 ± 169.1 dyn/cm/s−5, which was lower than the normal range (1200-1500 dyn/cm/s−5). The calculated SVR was significantly lower in the group with severe ventricular arrhythmia than in the group without severe ventricular arrhythmia (P < 0.05; Tables 2 and 3). Cardiac output, serum potassium, mean PA pressure, Child-Pugh classification, American Society of Anesthesiologists classification, etiology of cirrhosis, and number of patients with a prolonged QTc were similar in groups with severe ventricular arrhythmia and those with no arrhythmia during both insertion and removal of PA catheters.
| With Arrhythmia (n = 37) | Without Arrhythmia (n = 63) | P Value | |
|---|---|---|---|
| Mean arterial pressure (mm Hg) | 69.5 ± 10.4 | 74.5 ± 9.5 | 0.04 |
| CVP (mm Hg) | 9.6 ± 3.1 | 9.3 ± 3.0 | 0.61 |
| Cardiac output (L/minute) | 7.7 ± 2.0 | 7.2 ± 1.6 | 0.25 |
| SVR (dyn/cm/s−5) | 672.9 ± 175.6 | 750.0 ± 161.2 | 0.04 |
| Mean pulmonary artery pressure (mm Hg) | 19.5 ± 6.5 | 18.4 ± 5.8 | 0.40 |
| Potassium (mmol/L) | 4.10 ± 0.54 | 4.06 ± 0.62 | 0.74 |
| Time from right ventricle to pulmonary capillary wedge position (seconds) | 91.6 ± 103.6 | 53.3 ± 18.4 | 0.04 |
| QTc (milliseconds) | 438.5 ± 27.7 | 434.4 ± 23.1 | 0.43 |
| No. of prolonged QTc | 19 (51.4%) | 26 (42.0%) | 0.30 |
- NOTE: Values are reported as the mean ± standard deviation or number (%).
- Abbreviations: CVP, central venous pressure; prolonged QTc, rate-corrected QT interval > 440 milliseconds on lead II of the electrocardiogram; QTc, rate-corrected QT interval on lead II of the electrocardiogram [measured QT interval (seconds)/square root of RR interval (seconds)]; SVR, systemic vascular resistance [80(mean arterial pressure − CVP)/cardiac output].
| With Arrhythmia (n = 24) | Without Arrhythmia (n = 72) | P Value | |
|---|---|---|---|
| Mean arterial pressure (mm Hg) | 67.8 ± 10.3 | 74.4 ± 10.6 | 0.01 |
| CVP (mm Hg) | 10.9 ± 3.0 | 10.3 ± 2.5 | 0.39 |
| Cardiac output (L/minute) | 9.2 ± 2.5 | 8.5 ± 2.3 | 0.24 |
| SVR (dyn/cm/s−5) | 532.7 ± 168.0 | 655.8 ± 227.0 | 0.02 |
| Mean pulmonary artery pressure (mm Hg) | 22.1 ± 4.4 | 20.6 ± 4.4 | 0.17 |
| Potassium (mmol/L) | 4.06 ± 0.69 | 4.18 ± 0.54 | 0.41 |
| QTc (milliseconds) | 438.0 ± 22.3 | 435.2 ± 26.1 | 0.64 |
| No. of prolonged QTc | 13 (54.2%) | 29 (40.3%) | 0.19 |
- NOTE: Values are reported as the mean ± standard deviation or number (%).
- Abbreviations: CVP, central venous pressure; prolonged QTc, rate-corrected QT interval > 440 milliseconds on lead II of the electrocardiogram; QTc, rate-corrected QT interval on lead II of the electrocardiogram [measured QT interval (seconds)/square root of RR interval (seconds)]; SVR, systemic vascular resistance [80(mean arterial pressure − CVP)/cardiac output].
DISCUSSION
Several reports have suggested that arrhythmias are associated with the placement of a PA catheter in patients who are critically ill or undergoing cardiac surgery; the incidence in such patients varies from 13% to 70%.7, 8 Sprung et al.5 found that severe ventricular arrhythmia occurred in 53% of critically ill patients undergoing PA catheterization. They suggested that certain risk factors might predispose to the development of an arrhythmia, such as myocardial infarction, acidosis, and hypoxemia.5 Iberti et al.9 subsequently reported a 12.7% (7/56) incidence of severe ventricular arrhythmia in patients in an intensive care unit. The 37% incidence of severe arrhythmia found in this study is consistent with previous reports, despite the exclusion of patients with preexisting cardiac disease. In 4 patients (4.0%), intractable arrhythmias occurred, necessitating removal of the PA catheter; these arrhythmias were ventricular tachycardia associated with hemodynamic instability in 2 patients and ventricular fibrillation in the other 2 patients. The arrhythmias in these 4 patients were not corrected by administration of appropriate pharmacological agents, such as epinephrine and lidocaine, alone. However, removal of the catheter together with pharmacological therapy was associated with rapid restoration of sinus rhythm. In addition, a retrospective review of the patients' charts indicated that these 4 patients had developed transient ventricular tachycardia (3 patients) or ventricular fibrillation (1 patient) during insertion of the catheter. Therefore, intractable arrhythmia, unresponsive to pharmacological management during surgery, may be related to an indwelling PA catheter, particularly in patients who develop arrhythmia during catheter insertion.
A change in the pattern of the ECG, specifically prolongation of the QTc interval, has been observed in patients with alcoholic liver disease and in candidates for liver transplantation.10 QTc interval prolongation is but one index of the heterogeneity of refractoriness of abnormal ventricular function.11 This index has been proposed as a marker of the risk of arrhythmia after a myocardial infarction and as a marker of acute ischemia associated with atrial pacing.11 We suggest that in patients with end-stage chronic liver disease, prolongation of the QTc interval might be associated with re-entry or aberrancy of conduction. In addition, a recent report has suggested that patients with cirrhosis may develop chronotropic incompetence, electromechanical uncoupling, and QT interval prolongation.12 Abnormal indices of cardiac electrophysiology of unknown origin appear to be associated with an increased risk of sudden cardiac death in patients with advanced cirrhosis. It is unclear if a prolonged QTc interval subsides after liver transplantation.13, 14 In this study, 45% of patients had a prolonged QTc interval (>440 milliseconds), but the incidence was similar for groups of patients with and without arrhythmia (51.4% versus 42.0%).
Patients with end-stage chronic liver disease exhibit characteristic hemodynamic changes, which include increased cardiac output, tachycardia, and decreased SVR. However, the relatively small differences in the mean arterial pressure and SVR between patients with and without arrhythmias, despite the statistical significance of the differences, suggest that their clinical importance is minimal.
The development of arrhythmia during PA catheterization might be caused by mechanical trauma to the endocardium and prolonged irritation of the endocardium that might be influenced by the skill of the operator. This study has clearly demonstrated that increasing the time that a catheter is maintained in the right ventricle and/or PA is associated with a high frequency of arrhythmia, even though all PA catheterizations were supervised by 2 experienced attending anesthesiologists. It is possible that the sustained hyperdynamic circulation associated with end-stage chronic liver disease is one explanation for prolonged transit time. Enlargement of cardiac chambers in patients with end-stage liver disease, particularly the right ventricle, may contribute to prolonged transit time and eventually to the development of arrhythmia. However, this study did not estimate the size of cardiac chambers and did not demonstrate a relationship between the transit time and the size of cardiac chambers.
A high incidence of severe ventricular arrhythmia (53%) and a high mortality rate (51%) have been attributed to arrhythmias being precipitated by factors such as shock, hypoxemia, and acidosis.5 In this study, the severity of hypoxemia, acidosis, anemia, and abnormal serum electrolyte levels was similar in patients with and without arrhythmias (P > 0.05); indeed, values for these variables were close to normal.
A limitation of this study is that there was no control group of patients without cardiac and/or liver disease.
In conclusion, liver allograft recipients have a relatively high incidence of severe ventricular arrhythmias when the PA is catheterized. These arrhythmias are more often associated with mechanical irritation or trauma of the endocardium than with other patient factors.