Fluordeoxyglucose positron emission tomography contributes to management of pediatric liver transplantation candidates with fever of unknown origin
Abstract
Fever of unknown origin (FUO) frequently complicates the management of pediatric patients with terminal chronic liver failure during the pretransplantation period and may lead to increased morbidity and mortality. Nonhepatic origins of systemic infections may render the patient unsuitable for transplantation whereas infections within the liver may require organ resection for a cure. Therefore, accurate localization of the infection focus is critical for optimal management of children on the waiting list for liver transplantation. Here we report our experience using [18 F]fluordeoxyglucose (FDG)–positron emission tomography (PET) to detect the origin of infection in 11 children with biliary cirrhosis presenting with FUO during the waiting period for liver transplantation. In 5 children, positive intrahepatic FDG-PET signals correlated with bacterial cultures of the excised liver and/or anatomic or histologic signs of infection. Based on the FDG-PET findings, these patients underwent transplantation after continuous antibiotic treatment with ongoing, recurrent episodes of fever. In 6 children, no abnormal hepatic FDG-PET signals were found and no infections could be detected in the liver. Transplantation in these patients was performed only after becoming afebrile. Standard imaging techniques did not reveal abnormalities compatible with infection in any of the children. In conclusion, in children with biliary cirrhosis and FUO on the waiting list for liver transplantation, information obtained by FDG-PET imaging may be useful for decisions on therapy and suitability for liver transplantation. Liver Transpl 12:1698–1704, 2006. © 2006 AASLD.
Systemic infections are considered to be contraindications for liver transplantation.1 Therefore, pediatric candidates for liver transplantation presenting with febrile episodes need to be evaluated thoroughly. Fever of unknown origin (FUO) is defined as a temperature higher than 38.3°C on several occasions and lasting longer than 3 weeks, with a diagnosis that remains uncertain after 1 week of investigation.2 In patients awaiting liver transplantation, FUO often causes considerable delay in adequate treatment of serious infections, leading to deteriorated general condition of the transplant candidate and prolonged time and increased mortality on the waiting list. Sensitive, reliable diagnostic tools are needed to support a timely and accurate diagnosis of infection. In particular, the diagnosis of an intrahepatic focus of infection is important for the management of the candidate on the waiting list, as the presence of a localized intrahepatic infection without systemic involvement does not render the patient unsuitable for transplantation. In the absence of overt pathology, e.g., abscess formation reliably identified by ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI), alternative modes of imaging, e.g., scintigraphy, have been tested to adequately identify a focus of intrahepatic infection. In general, 3 scintigraphic methods have been applied. The use of 67Ga-citrate has been largely abandoned because of unfavorable dosimetry and image quality. The alternative has become radiolabeled autograft leukocytes. 111In-labelled (isotope labeling with 111Indium) or 99mTc-labeled (isotope labeling with 99mTechnetium) leukocytes (white blood cells) are routinely used in clinical practice. However, they have limitations, such as instability of the labeling and high radiation burden,3 the cumbersome collection of blood in pediatric patients, and the relatively long time between injection and diagnosis. In the recent years, [18 F]fluordeoxyglucose (FDG)–positron emission tomography (PET) has become a standard procedure in the workup of patients with oncological diseases.4 The high sensitivity of FDG-PET coincides with a relatively low specificity, due to accumulation of FDG in inflammatory processes.3 This drawback has, however, led to a new development, i.e., the use of FDG-PET in inflammatory conditions including FUO. PET has been known for some time to be useful in localization and imaging of infectious foci.5-8 Infection imaging with FDG-PET relies on the fact that granulocytes and mononuclear cells use glucose as an energy source specifically during their metabolic burst upon activation by triggers.3, 9 Therefore, understandably, FDG accumulates in many different types of inflammatory lesions as well as in malignant tumors. The advantages of FDG-PET are early imaging after injection,8 higher resolution and higher target-to-background ratio,3 sensitivity to chronic low-grade infections,3, 6, 10, 11 and high interobserver agreement.4, 10, 12 Here we present our experience using FDG-PET to positively identify intrahepatic infections in children on the waiting list for liver transplantation while other imaging modalities failed. FUO is considered a contraindication for transplantation; however, hepatic infections are frequently only cured by removal of the infected organ during transplantation. Therefore the potential of FDG-PET to identify intrahepatic infections may be crucial for management of patients with FUO awaiting liver transplantation.
Abbreviations
FDG, [18 F]fluordeoxyglucose; PET, positron emission tomography; FUO, fever of unknown origin; CT, computed tomography; MRI, magnetic resonance imaging; BA, biliary atresia.
MATERIALS AND METHODS
Patients
From January 2003 to January 2006, 37 children underwent liver transplantation in the University Medical Center Groningen. A total of 11 of these patients presented with FUO and were included in the study (for details see Table 1). All patients suffered from terminal liver failure, were listed for liver transplantation, and presented with FUO before FDG-PET imaging studies were performed. In none of the patients did clinical investigations or standard imaging (ultrasound and/or CT or MRI) enable identification of a focus of infection (e.g., abscesses). Laboratory investigations at the time of FDG-PET imaging revealed elevated C-reactive protein serum concentrations in all patients (median 46 mg/L, normal range <5 mg/L) and leukocytosis in 6/11 patients (median 21.5 × 103 /L). Eight patients previously had splenomegaly associated with thrombocytopenia and leukopenia. Blood and urine cultures were repeatedly taken from all patients in the period before and after FDG-PET examination (range 8-17 blood cultures per patient in 3 months previous to FDG-PET). Within 2 weeks before FDG-PET examination none of the cultures revealed bacteremia or urinary tract infection. For the purpose of this study we defined true-positive FDG-PET findings as intrahepatic PET signals with a greater degree of uptake than surrounding liver and correlating with positive results from cultures taken intraoperatively during the transplantation procedure in the liver or perihepatic area and/or pathology or histology of the resected liver indicating focal infection. True-negative findings were defined as FDG-PET uptake to a degree not higher than surrounding cirrhotic liver along with negative cultures taken intraoperatively and pathology of the resected organ being compatible with biliary cirrhosis only, without any signs of focal infection or inflammation.
| Patient number | Gender | Diagnosis | Age at LT | PET signal in liver | PET signal extrahepatic | Pathology explant | Culture explant (e), ascites (a), bile (b), urine (u) |
|---|---|---|---|---|---|---|---|
| Patients with positive intrahepatic FDG-PET signals | |||||||
| 1 | F | BA | 2 yr 11 months | Multiple intrahepatic lesions, segment 4 diffusely affected | Pharynx, femur | Infectious necrosis liver parenchyma segment 4, biliary stasis | Enterococcus faecium (e) |
| 2 | M | BA | 1 yr 8 months | Focus segments 2 and 4 | None | Abscess segment 4 | S. epidermidis (e) |
| 3 | M | PSC | 9 yr 10 months | Focus segment 8 | None | NFL | Corynebacteria (e) |
| 4 | M | BA | 8 months | Left lobe | Diaphragm | NFL | Enterococcus faecium ((a) at liver resection) |
| 5 | M | BA | 10 months | Diffuse, compatible with multilocular cholangitis | None | Micro-abscess formation in bile ducts, suppurative cholangitis | S. epidermidis (e) |
| Patients with negative intrahepatic FDG-PET signals* | |||||||
| 6 | M | NsPBD | 9 yr 7 months | Negative | None | NFL | Negative |
| 7 | F | BA | 2 yr 1 months | Negative | None† | NFL | Negative |
| 8 | M | CLF | 7 yr 1 months | Negative | Gallbladder | NFL | Negative |
| 9 | M | BA | 1 yr | Negative | Urinary tract | NFL | Negative |
| 10 | F | BA | 6 months | Negative | Retroperitoneal | NFL | Negative |
| 11 | M | PFIC | 1 yr 8 months | Negative | Retention in ureter, pharynx | NFL | Enterobacter cloacae (u) |
- * FDG-PET signals isointense with surrounding liver, see definition in “Patients” section and Figure 1a and b.
- † Area actually void of signal in the brain (Fig. 1c).
- Abbreviations: LT, liver transplantation; NFL, no focal lesions compatible with inflammation, abscess or infection; NsPBD, nonsyndromic paucity of bile ducts; PSC, primary sclerosing cholangitis; PFIC, progressive familial intrahepatic cholestasis; CLF, congenital liver fibrosis.
PET Methodology
The application of FDG-PET in the pediatric patients followed a simple scheme. The patient was injected intravenously with a dose of 4-5 megabecquerel (MBq)/kg body weight, followed by a waiting period of 60 to 90 minutes. After this waiting period the patient was scanned from the thighs upward, in a Siemens Ecat Exact HR+ (Siemens, Knoxville, TN) scanner, using an interleaved protocol for emission and transmission scanning (alternating emission and transmission). On average, data acquisition for 1 bed position took between 3 and 7 minutes. The axial field of view of the camera determines the number of bed positions needed, and thus the total length of the scan. The HR+ cameras have an effective field of vision of just over 15 cm, resulting in 4 to 5 bed positions needed in children, resulting in a total period of up to approximately 30 minutes needed for scanning.
RESULTS
Five of 11 patients had abnormal FDG-PET results with increased focal uptake in liver parenchyma as compared to surrounding cirrhotic tissue. In all 5 patients undergoing liver transplantation with previously detected intrahepatic positive FDG-PET signals, findings in the explanted liver were abnormal (Table 1). Structures were identified that were macroscopically or histologically compatible with local infections (patients 1, 2, and 5) or cultures taken intraoperatively from the explanted organ grew bacterial strains, which may infect chronically diseased biliary trees in cirrhosis (patients 1-5). Thus, all of these FDG-PET findings were considered true-positives. In 6 patients with FUO, FDG-PET scans did not reveal any increased focal uptake. The uptake was isointense on background level throughout the whole organ. In these patients' cultures, macroscopic or microscopic findings at the time of transplantation did not reveal signs of focal infection. These FDG-PET results were considered true-negatives.
All 11 patients in this study suffered from biliary cirrhosis.
Clinical Characteristics of Patients With FUO and True-Positive Intrahepatic FDG-PET Findings
All patients with positive intrahepatic FDG-PET results were diagnosed with primary biliary-tract diseases: 4 of 5 patients had been diagnosed with biliary atresia (BA) and had undergone a Kasai operation (portoenterostomy); 1 patient had primary sclerosing cholangitis. All patients with BA had experienced 1 or more bouts of cholangitis earlier in the disease course. One patient (diagnosed with BA) had received steroids in the postoperative course after a Kasai operation. Patient 3 with primary sclerosing cholangitis had been treated for cholangitis, hydropic gallbladder, and renal failure 6 weeks before transplantation. Following bile duct decompression through papillotomy, he recovered clinically but developed persistent fever in the later course. Ultrasound and/or CT or MRI were performed in all patients and revealed irregular, partially dilated bile ducts in 1 (patient 3) and 3 inhomogeneous cystic lesions in the right and the left liver lobe in another patient (patient 1), which were found to accumulate FDG-PET signal. None of these lesions showed signs of an abscess in standard imaging. In the period of 3 months to 2 weeks before FDG-PET was performed, E. coli bacteremia was detected in patient 2, for which he received antibiotics according to susceptibility. Bile was cultured in 1 case and revealed a streptococcus species coinciding with biliary obstruction, which was treated with decompression (patient 3) and antibiotics according to susceptibility. Patients 2 and 3 recovered initially following antibiotic treatment. Febrile episodes recurred, however, necessitating further diagnostic procedures. Repeatedly, an enterococcus species was cultured in feces from 1 patient (patient 1). Single or multiple intrahepatic FDG-PET signals were detected in segments 2, 4, and 8 and diffusely in the left liver lobe (Table 1; Figs. 1 and 2). Superposition of FDG-PET and previously taken MRI images allowed a co-localization of presumed sites of infection with irregularly distended bile ducts (patient 3; Fig. 2). Sites of physiologic tracer accumulation included brain, kidneys, bladder, and frequently sites of increased muscle activity, e.g., the diaphragm and perilingual regions (patient 4). Positive, isolated, intrahepatic FDG-PET signals were interpreted as potential cholangitis and treated with broad-spectrum antibiotics until liver transplantation.
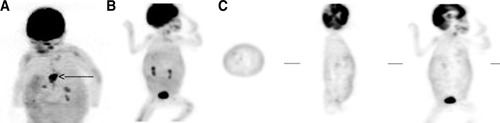
(A) Positive FDG-PET scan in a 2-yr-old girl with BA and a Kasai operation, presenting with fever of unknown origin. This patient (patient 1) became increasingly cholestatic in the second year of life. She developed signs of portal hypertension and presented with recurrent episodes of fever. Ultrasound and MRI detected diffuse, cyst-like dilatations of bile ducts. FDG-PET revealed increased uptake in multiple areas of the liver with significant accumulation in segment 4 (arrow). In the explanted liver this structure was identified as infectious parenchymal necrosis; in cultures, Enterococcus faecium was detected and the patient was treated according to susceptibility after transplantation. At 2 yr after transplantation, the child is well. Note the uptake of tracer in periorbital and perilingual regions. The pharynx was inspected repeatedly for infectious foci, which could not be identified. (B,C) Negative FDG-PET scan of a patient with no signs of hepatic infection (patient 7). (B) Shows the maximum intensity projection. (C) Shows tomographic images in the transverse, sagittal, and coronal plane. The hepatic uptake is isointense throughout the whole organ (B,C). Note the normal excretion via the kidneys. The patient had suffered from a cerebrovascular accident, which is visible as an area void of signal in the brain (C).
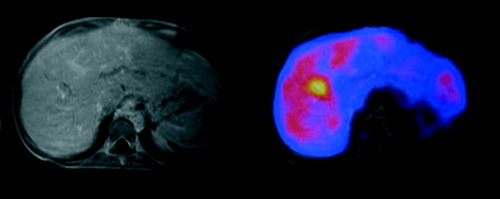
Superposition of FDG-PET and MRI images demonstrates a colocalization of the suspected infectious focus with dilated bile duct structures. A 15-yr-old boy (patient 3), diagnosed with primary sclerosing cholangitis (PSC), was on the waiting list for liver transplantation when he developed recurrent bouts of fever, 6 weeks after recovery from acute cholangitis and cholecystitis. For the latter, he was treated with endoscopic decompression and antibiotic treatment according to susceptibility of streptococcus found in bile culture. At the time of performing FDG-PET, ultrasound did not show any focal infectious lesions, MRI of the liver revealed irregular bile ducts with dilatations and strictures compatible with PSC. FDG-PET showed uptake in a single area in segment 8 of the liver.
Four patients underwent liver transplantation during periods of recurrent fever due to presumed cholangitis without signs of systemic sepsis. In 1 patient fever did not recur after long-term treatment with antibiotics. Intra- and postoperatively, all patients received antibiotic treatment according to susceptibility testing of the detected strain. One patient died following primary nonfunction of the graft. Three patients are now in good clinical condition, 2 in their second yr after liver transplantation. In 1 patient, biliary strictures occurred postoperatively that required transient stenting 2 months after liver transplantation.
Clinical Characteristics of Patients With FUO and True-Negative Intrahepatic FDG-PET Scans
Five of 6 patients were diagnosed with diseases primarily affecting the bile ducts. Three patients had BA, 1 patient each had congenital hepatic fibrosis, nonsyndromic paucity of bile-ducts, and progressive familial intrahepatic cholestasis, respectively. In none of the patients did clinical examination or ultrasound- or CT imaging reveal a focus for infection. Two patients had a nonphysiologic accumulation of FDG-PET signal in the urinary-tract system (patients 9 and 11), 1 of whom subsequently had a positive urine culture revealing Enterobacter infection (patient 11). Both patients were treated for urinary tract infection and recovered. Four patients recovered from recurrent fever after empiric antibiotic therapy before undergoing transplantation. None of the patients had fever at the time of transplantation. In none of the patients did macro- or histopathology of the explanted liver or intraoperative perihepatic cultures show focal signs of infection.
DISCUSSION
Clearly, morbidity and mortality before and after liver transplantation correlate with frequency and severity of infections of hepatic and extrahepatic origin.13 Cholangitis is a frequent complication in patients with biliary cirrhosis awaiting liver transplantation. For detection of hepatic infections diagnostic tools are limited. Positive identification of infections such as cholangitis in the liver parenchyma may be difficult and diagnoses are frequently made on the basis of exclusion.
Focal infectious processes can be detected by several radiological techniques, including CT, MRI, and ultrasound. However, positive identification of infectious and inflammatory foci may be difficult in their early phases because of the lack of substantial anatomical changes at that time. Also, discrimination of active infectious or inflammatory lesions from residual changes due to chronic disease (e.g., liver cirrhosis) or surgery remains difficult. All of our patients presented with elevated levels of serum C-reactive protein as an indicator of ongoing infection. Other parameters such as procalcitonin have been shown to be advantageous in discriminating infectious from inflammatory foci14 and may also be of value in patients with liver cirrhosis and FUO. Risk of infection of the biliary tract is high in patients after Kasai procedure due to BA15-17 and may be observed in patients with strictures of the biliary tract such as in primary sclerosing cholangitis.13, 18 Recurrent biliary infections in these diseases are considered to be risk factors for deterioration of liver function prior to liver transplantation.13 FUO is considered a relative contraindication, and systemic infections should be treated before liver transplantation. However, infection originating from within the liver may not be curable without removal of the diseased organ.1 For positive identification of patients who are potential candidates for liver transplantation in spite of recurrent fever episodes during ongoing localized hepatic infections, sensitive tools for diagnosis are mandatory. In the patients presented here, imaging by ultrasound, CT, or MRI had failed to demonstrate infectious lesions in the cirrhotic liver. Although the number of patients in our retrospective study is limited, our results confirm those from other studies that FDG-PET is a sensitive test for detecting infectious foci in FUO.2, 19 When correlated with anatomic or histologic signs of hepatic infections and/or intraoperative cultures of the excised liver, all 5 abnormal intrahepatic FDG-PET signals can be considered true-positives. We cannot exclude that Corynebacterium and Staphylococcus epidermidis, identified in intraoperative cultures from explanted livers (patients 2, 3, and 5), are contaminants. However, both strains have been described as opportunistic pathogens leading to hepatic abscesses in immunocompromised patients.20, 21 The abscesses found in the resected liver of patients 2 and 5 underscore the potential role of staphylococcus as a liver pathogen in patients with biliary cirrhosis. An obvious problem of FDG-PET imaging is low specificity.22 This is demonstrated in patients with positive FDG-PET signals originating from extrahepatic structures. In this case, differentiation between tissue with physiologic uptake (e.g., muscle tissue) and enhanced signal due to infection may be difficult. In a single case, culture results correlated with signal accumulation in the urinary tract. In patients with positive FDG intrahepatic signals and thus suspected hepatic infections, antibiotic treatment was continued until transplantation while the patient had no signs of systemic involvement of infection, except for recurrent febrile episodes. Based on the FDG-PET result the patients remained active candidates on the waiting list and underwent liver transplantation. During the immediate postoperative period and initiation of immunosuppressive therapy no complications arose from systemic infection after removal of the affected organ. The number of patients examined in our study is too small to conclude whether this approach can be considered as safe. Further studies are needed to address the risks of performing liver transplantation in this setting. However, as we think that positive detection of intrahepatic infectious lesions has strong implications for management of these patients with regard to their suitability for transplantation, we propose an algorithm for diagnostic procedures for pretransplantation patients with FUO as depicted in Figure 3. This algorithm should be tested in larger studies to confirm the role of FDG-PET in febrile patients with cirrhosis. In conclusion, FDG-PET may contribute to optimized management of pediatric patients with FUO on the waiting list for liver transplantation. However, extrahepatic signal accumulation must be interpreted with caution. Further prospective controlled studies are warranted to evaluate FDG-PET as a diagnostic tool to identify hepatic infection in liver transplant candidates with FUO.
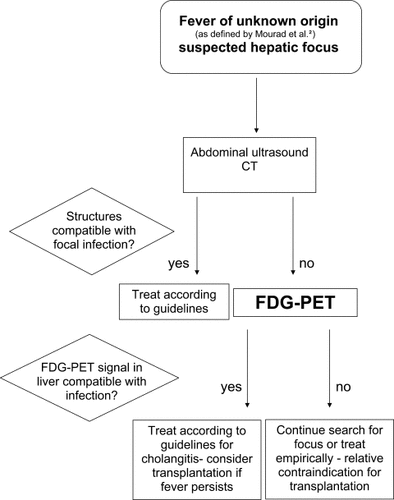
Proposed algorithm for diagnostic imaging procedures including FDG-PET in pediatric liver transplantation candidates with fever of unknown origin and suspected hepatic infection.
Acknowledgements
We thank Eric J. van der Jagt (Department of Radiology, University Medical Center Groningen) for expert advice in interpretation of radiological imaging.




