Ischemia-reperfusion of rat liver modulates hepcidin in vivo expression
Abstract
The recently identified acute-phase response antimicrobial peptide hepcidin has been postulated to maintain iron homeostasis by modulating iron absorption at both the intestinal and macrophage levels. Hepcidin has also been reported to be responsible for anemia associated with chronic inflammatory diseases, and in anemia in patients with hepatic adenomas. Since Kupffer cells are known to be the primary contributor to early-phase ischemia-reperfusion injury in the liver and iron is known to modulate Kupffer cell production of proinflammatory cytokine and reactive oxygen species, we investigated hepcidin in vivo expression in the well-established rat partial-liver ischemia-reperfusion model. We found that both liver ischemia alone and liver ischemia-reperfusion significantly induced serum and liver hepcidin levels. Furthermore, currently proposed mediators of in vivo hepcidin expression, such as interleukin-6, signal transducers and activators of transcription-family transducers, and CCAAT/enhancing binding protein-alpha do not appear to modulate hepcidin expression in the liver ischemia-reperfusion acute inflammatory model. In this study we report the first in vivo evidence of liver ischemia and liver ischemia-reperfusion modulation of hepcidin expression. In conclusion, in the well-characterized liver ischemia-reperfusion model of acute inflammation, mechanism(s) other than interleukin-6 signal transduction via signal transducers and activators of transcription-3 may be responsible for hepcidin induction. (Liver Transpl 2005;11:800–806.)
The recently identified modulator of iron metabolism, hepcidin, has been postulated to maintain iron homeostasis by regulating iron absorption at the crypt cell level by inhibition of iron uptake, and at the macrophage/Kupffer cell (KC) level by inhibition of iron release. These hepcidin-dependent effects are believed to be responsible for anemia seen in chronic inflammatory diseases. Hepcidin expression has also been shown to be mediated by iron overload (acute, dietary), and under acute inflammatory states.1-4
Hepcidin has been reported to be induced in livers of mice under conditions of iron overload1, 4 and abrogated iron overload in mice under experimental hemochromatosis.5 In contrast, hepcidin expression is suppressed under conditions of anemia (bloodletting and iron-poor diet).6 Hepcidin's role in humans is somewhat controversial, since refractory anemia was seen in humans with liver adenomas, which were reported to over express hepcidin7; however, mutations in the hepcidin gene were identified in patients with hemochromatosis.8
Pigeon et al. reported that hepcidin messenger RNA (mRNA) does not contain iron-responsive elements,1 structural elements known to be present in transcript of major mediators of iron metabolism such as ferritin and the transferrin receptor.9, 10 However, Courselaud et al. recently reported that liver hepcidin expression might be regulated by the CCAAT/enhancer-binding protein alpha (C/EBPα).11 Although the hepcidin gene has been reported to be one of the most prominently expressed genes in adult liver, the understanding of its regulation is largely unknown.11 Therefore, further studies are required to understand how hepcidin expression is modulated under various inflammatory states. Under inflammatory conditions, hepcidin expression may reduce or enhance tissue injury. To this end, and given that (1) iron levels mediate KC production of proinflammatory cytokines and reactive oxygen species,12, 13 (2) KCs are primarily responsible for early-phase liver injury due to ischemia-reperfusion (I/R),14 (3) hepcidin has been postulated to control iron metabolism at the macrophage/KC level,15 and (4) hepcidin has been reported to be induced under acute inflammatory states,6 we investigated hepcidin expression in liver subjected to ischemia-reperfusion. In this study, we report the first evidence of the modulation of hepcidin expression by liver ischemia alone, and by liver I/R, and that in vivo hepcidin expression might not be modulated by interleukin (IL)-6, signal transducers and activators of transcription (STAT)-family signal transducers, or C/EBPα in the liver I/R acute inflammatory model.
Abbreviations
KC, Kupffer cell; mRNA, messenger RNA; C/EBPα/β, CCAAT/enhancing binding protein alpha/beta; I/R, ischemia-reperfusion; IL, interleukin; STAT-1, -3, and -5, signal transducers and activators of transcription-1, -3, and -5; GAPDH, glyceraldehyde phosphate dehydrogenase.
Materials and Methods
Animals
Male Sprague-Dawley rats (200–250 g) were purchased (Charles Rivers, Houston, TX). All animals used in this study received a nutritionally-balanced rodent diet with “normal” iron level (∼35 mg iron/kg diet), and water ad libitum. Animals were cared for according to National Institutes of Health guidelines.
Warm Partial-Hepatic I/R Model
Liver warm in vivo I/R injury was achieved as previously described.16 In brief, animals were anesthetized with Nembutal (50–60 mg/kg body weight, i.p., Sigma-Aldrich, St. Louis, MO), and under aseptic conditions, a laparotomy was performed to access the liver. Partial-warm in vivo hepatic ischemia was produced in the experimental group by placement of vascular microclips across the hilum of the median and left lateral lobes for 45 minutes. In the group receiving liver ischemia alone, after 45 minutes of ischemia, blood and liver samples were collected without removal of the microclip. In rats treated with liver ischemia and reperfusion, liver ischemia was conducted as described above, followed by the removal of microclips to produce 60 minutes of reperfusion, but before collecting samples. Sham-control animals received saline, anesthestic, and laparotomy only.
Serum Hepcidin
Serum (50 μL) hepcidin levels in rats were measured by enzyme-linked immunosorbent assay, according to the manufacturer's protocol (EIA-4015, DRG International, Mountainside, NJ).
Immunohistochemical Analysis of Liver Hepcidin Protein
Five-micron paraffin-embedded liver sections were hydrated and treated with 3% hydrogen peroxide in methanol to inhibit endogenous peroxidases. A hepcidin primary antibody (diluted 1:200, HEPC11-A, ADI International, San Antonio, TX) was used to probe sections for hepcidin. Subsequently, a goat anti-rabbit biotinylated immunoglobulin G secondary antibody (diluted 1:400, VECTOR Laboratories, Inc., Burlingame, CA) were used for immunodetection and visualization of hepcidin, with the horseradish peroxidase substrate diaminobenzidine. Sections were counterstained with Harris hematoxylin. The specificity of staining was assessed by exclusion the primary antibody in the staining process.
Reverse Transcription-Polymerase Chain Reaction Analysis of Liver Hepcidin mRNA
Total RNA was extracted from liver tissue using an UltraSpec Total RNA Isolation Kit (BL-10050, Biotecx Laboratories Inc., Houston, TX). Complementary DNA was transcribed with 4 μg of total RNA, random hexamers, and a SuperScript II Preamplification System (18089-011, GIBCO BRL, Life Technologies, Grand Island, NY) per manufacturer's protocol. Using specific primers for hepcidin and glyceraldehyde phosphate dehydrogenase (GAPDH), their complementary DNAs were amplified by polymerase chain reaction under the following conditions: hepcidin (26 cycles, 94°C for 60 seconds, 55°C for 60 seconds, and 72°C for 120 seconds), and GAPDH, (28 cycles, 94°C for 60 seconds, 52°C for 60 seconds, and 72°C for 60 seconds). Polymerase chain reaction primers (Sigma-Genosys, Woodlands, TX) used were as follows: hepcidin forward primer (5′- CACGAGGGCAGGACAGAAGGCAAG-3′) and hepcidin reverse primer (5′- CAAGGTCATTGCTGGGGTAGGACAG-3′) to give a 412-bp product, GAPDH forward primer (5′-GCCAAGTATGACATCAA-3′) and GAPDH reverse primer (5′-CCATATTCATTGTCATACCA-3′) to give a 203-bp product. All polymerase chain reaction products were electrophoresed on 2% agarose gels (Fisher Scientific, Fair Lawn, NJ). Bands were visualized by poststaining for 30 minutes with GelStar Nucleic Acid Gel Stain (FMC Bioproducts, Rockland, MA), and photographed. Photographs were digitized and evaluated as stated above. The relative expression of hepcidin mRNAs were assessed by taking the ratio of the intensity of the DNA bands of hepcidin and to GAPDH band, and then expressed as arbitrary units. To ensure an equal amount of RNA was used for all samples, RNA concentration was determined spectrophotometrically, and its integrity evaluate on agarose gel. DNA bands were digitized (Corel Photohouse 2.0, Ontario, Canada) and evaluated using image analysis software (Scion Image Beta 3b, NIH Image modified for Windows by Scion Corp., Frederick, MD).
Serum Iron
Total iron content in serum was determined as previously described.17 This method involved precipitating the proteins in serum, reducing all iron to Fe2+, complexing Fe2+ with ferrozine, and measuring the optical density of the ferrozine-Fe2+ complex spectrophotometrically at 562 nm. All reagents were prepared in Chelex 100 (Sigma, St. Louis, MO)–treated deionized water, and all plastic and glassware was rinsed with 5 mmol/L EDTA prepared in Chelex 100-treated deionized water. All measurements employing ultraviolet/visible spectrometry were done on a DU 640B spectrophotometer (Beckman Instruments, Inc., Fullerton, CA).
Western Blot Analysis of Liver C/EBPα and STAT-1, -3, & -5
Liver cytoplasmic fraction in Tris-EDTA (10 mmol/L Tris-HCl, 1 mmol/L EDTA, 0.1 mmol/L phenylmethol sulfonyl fluoride, and cØmplete protease inhibitor, pH 7.9) buffer, containing cytoplasmic C/EBPα proteins, and liver nuclear fraction in Tris-sodium chloride-EDTA (50 mmol/L Tris-HCl, 420 mmol/L NaCl, 1 mmol/L EDTA, 0.1 mmol/L phenylmetholsulfonyl fluoride, and cØmplete protease inhibitor, pH 7.9) containing C/EBPα and STAT-1/3/5 nuclear proteins were isolated as described by Rudiger et al.18 and used for Western blot analysis. Protein (27 or 100 μg) was separated on NuPAGE (Carlsbad, CA) gels (10%) and transferred to nitrocellulose membranes (Schleicher and Schuell, Keene, NH). Protein bands were probed with a C/EBPα polyclonal antibody (diluted 1:200, Active Motif, Carlsbad, CA) and an anti-rabbit secondary antibody. Equal loading and transfer to the membranes was confirmed by staining the blots with Ponceau S (Sigma, St. Louis, MO). Membranes were immunoblotted for β-actin with an affinity purified goat polyclonal antibody diluted 1:500 (sc-1616, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and a horseradish peroxidase–conjugated anti-rabbit secondary antibody (sc-2350, Santa Cruz Biotechnology, Inc.) (diluted 1:1000). Actin bands were visualized with a commercially available enhanced chemiluminescence kit (Amersham Life Sciences, Piscataway, NJ). C/EBPα and actin bands were digitized using Scion Image Beta 3b software (Scion Corp.), and densitometric values for C/EBPα bands normalized using densitometric values obtained for actin bands. Western blot analysis for cytoplasmic and nuclear STAT-1, -3, and -5 was performed as described for C/EBPα above, except that commercially available primary antibodies specific to phosphorylated and nonphosphorylated STAT-3 were employed.
Serum IL-6 Levels
IL-6 levels was measured using commercially available enzyme linked immunosorbent assay kits (ER2IL-62, Pierce Endogen, Rockford, IL) for the rat IL-6.
Protein Concentration
All protein concentrations were determined according to the method of Lowry et al.19
Statistics
All data were analyzed by ANOVA, and subsequently subjected to Bonferroni t test or Holm-Sidak method to determine significant differences between groups.
Results
Serum Aspartate Aminotransferase and Lactate Dehydrogenase Activities as a Measure of Liver Injury
No significant difference in serum aspartate aminotransferase or lactate dehydrogenase activity was found between the 45I/0R group and sham-control group (92.6 ± 21.1 U/L vs. 39.4 ± 2.0 U/L, and 1314.3 ± 522.5 U/L vs. 1003.8 ± 280.2 U/L, respectively). In contrast, significant increases in serum aspartate aminotransferase and lactate dehydrogenase activities were found in the 45I/60R group, compared with the sham-control and 45I/0R groups (317.6 ± 39.8 U/L vs. 39.4 ± 2.0 U/L and 92.6 ± 21.1 U/L, P < 0.00002 and P = 0.0002, respectively), and (3927.2 ± 794.2 U/L vs. 1003.8 ± 280.2 U/L and 1314.3 ± 522.5 U/L, P = 0.011 and P = 0.018, respectively).
Serum Hepcidin
In this study, enzyme linked immunosorbent assay analysis of serum hepcidin, we were unable to detect hepcidin in serum of the sham-control group (Fig. 1). Significant increases in serum hepcidin were found in animals subjected to liver ischemia alone or ischemia-reperfusion, compared to the sham-control group, P = 0.024 and P = 0.009, respectively (Fig. 1).
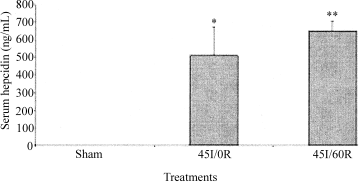
Enzyme linked immunosorbent assay analysis of serum hepcidin after warm in vivo liver ischemia-reperfusion. Sham-control animals received saline, anesthetic, and laparotomy only, and livers of other groups were subjected 45 minutes of ischemia alone (45I/0R), or 45 minutes of ischemia plus 60 minutes of reperfusion (45I/60R), in vivo. Serum (50 μL) hepcidin levels in rats were measured by enzyme linked immunosorbent assay, according to the manufacturer's protocol. Data are expressed as the mean ± SEM (n = 3). *P = 0.029 compared to sham-control animals. **P = 0.009 compared to sham-control animals.
Immunohistochemical Detection of Liver Hepcidin
Liver sections from sham-control rats showed minimal staining for hepcidin around very few blood vessels (Fig. 2A). In contrast, liver sections from rat subjected to liver ischemia alone had more intense staining for hepcidin around many blood vessels observed in the sham-control liver sections (Fig. 2B). However, in rats subjected to liver ischemia-reperfusion, more intense staining for hepcidin was observed compared to sham-control liver section (Fig. 2C). In addition, a significantly greater number of hepatocytes stained for hepcidin, and staining was not localized only to hepatocytes adjacent to blood vessels, but to hepatocytes in all zones of the liver (Fig. 2C).
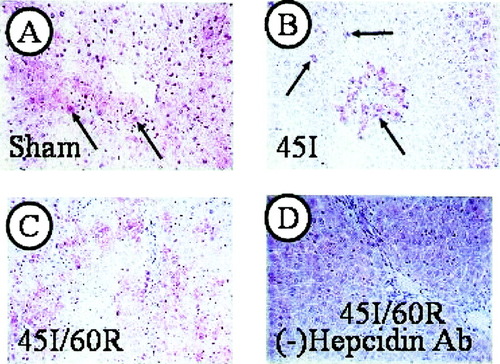
Immunohistochemical analysis of liver hepcidin after warm in vivo ischemia-reperfusion. Sham-control animals received saline, anesthetic, and laparotomy only, and livers of other groups were subjected 45 minutes of ischemia alone (45I/0R), or 45 minutes of ischemia plus 60 minutes of reperfusion (45I/60R), in vivo. At the end of each experiment, livers samples were placed in buffered formalin, 5-μm sections were made, and immunohistochemistry was performed as described in Materials and Methods. Stained sections were examined by light microscopy (original magnification, ×25). (A) Sham-control liver with light stainings for liver hepcidin around liver venules (solid arrow). (B) Evidence of a greater number and more intense staining for hepcidin in 45I/0R livers. Arrows indicate hepatocytes staining for hepcidin. (C) Significantly greater and more general staining of hepatocytes in livers subjected to 45I/60R. (D) A minus antibody control (with a 45I/60R liver section), to demonstrate the specificity primary antibody.
Hepcidin mRNA Levels
Constitutive expression of hepcidin mRNA was found in livers sham-control animals (Fig. 3A). A significant increase in liver hepcidin mRNA was found in animals subjected to liver ischemia and reperfusion, compared to liver hepcidin mRNA levels in sham-control animals, P < 0.004 (Fig. 3B). Similarly, a significant increase in liver hepcidin mRNA was found in animals subjected to liver ischemia-reperfusion, compared to liver hepcidin mRNA levels in animals subjected liver ischemia alone, P = 0.034 (Fig. 3B). In contrast, although liver hepcidin mRNA level increased in rats whose livers were subjected to ischemia alone, it did not reach statistical significance, compared to hepcidin mRNA level in livers of sham-control rats (Fig. 3B).
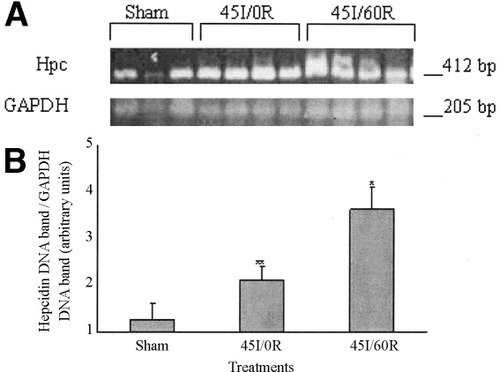
(A) Reverse transcription-polymerase chain reaction analysis of hepcidin mRNA levels in rat liver after warm in vivo ischemia-reperfusion. Liver hepcidin mRNA level was determined by semiquantitive reverse transcription-polymerase chain reaction using liver total RNA (2 μg). Sham-control animals received saline, anesthetic, and laparotomy only, and livers of other groups were subjected 45 minutes of ischemia alone (45I/0R), or 45 minutes of ischemia plus 60 minutes of reperfusion (45I/60R), in vivo. Polymerase chain reaction products were run on agarose gels, and gels stained with GelStar. (B) Gels were photographed and digitized, and optical densities of hepcidin mRNA bands were normalized using optical densities of the GAPDH mRNA bands. Values are expressed as mean ± SEM, (n = 3–4) of arbitrary units. *P = 0.004 compared to sham-control animals. **P = 0.034 compared to 45I/0R animals
Serum Iron
A significant decrease in serum iron was found in rats subjected to liver ischemia or liver ischemia-reperfusion, compared to serum iron of sham-control animals, P < 0.001 and P = 0.007, respectively (Fig. 4).
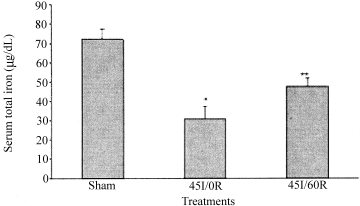
Total serum iron following warm in vivo liver ischemia-reperfusion. Sham-control animals received saline, anesthetic, and laparotomy only, and livers of other groups were subjected 45 minutes of ischemia alone (45I/0R), or 45 minutes of ischemia plus 60 minutes of reperfusion (45I/60R), in vivo. Iron in aliquots (250 μL) of serum was reduced to Fe2+, complexed to ferrozine, and determined spectrophotometrically at 562 nm. Values are expressed as mean ± SEM (n = 5–6). *P < 0.001 compared to sham-control animals. **P = 0.007 compared to sham-control animals.
Activation of Liver STAT-1,-3, and -5 and C/EBPα
No significant changes in liver nuclear phosphorylated STAT-3 (pSTAT-3) and STAT-3 was found in this study between groups (Fig. 5). Similar results were found for both STAT-1 and STAT-5 also (data not shown). In this study we investigated C/EBPα levels in both nuclear and cytoplasmic extracts by Western blot, but we could not detect C/EBPα in nuclear extracts despite significantly increasing the amount of nuclear extract protein. However, C/EBPα was readily detected in the cytoplasmic fraction. Although a decline in cytoplasmic C/EBPα was observed in the 45I/0R group, it did not reach statistically significant levels, and it returned to sham levels by 60 minutes of reperfusion (Fig. 6).
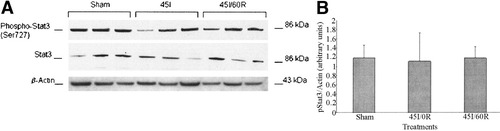
(A) Western blot analysis of pSTAT-3 following warm in vivo liver ischemia-reperfusion. Sham-control animals received saline, anesthetic, and laparotomy only, and livers of other groups were subjected 45 minutes of ischemia alone (45I/0R), or 45 minutes of ischemia plus 60 minutes of reperfusion (45I/60R), in vivo. Liver nuclear fraction in Tris-sodium chloride-EDTA (50 mmol/L Tris-HCl, 420 mmol/L NaCl, 1 mmol/L EDTA, 0.1 mmol/L phenylmethol sulfonyl fluoride, and cØmplete protease inhibitor, pH 7.9) containing pSTAT-3 nuclear protein was used for Western blot analysis. Protein (27 μg) was separated on NuPAGE gels (10%) and transferred to nitrocellulose membranes. Western blot analysis was done as described in Materials and Methods. (B) Densitometry of pSTAT-3 Western blot was performed as described in the legend to Figure 3. No statistically significant difference in liver pStat-3 was detected between groups. Data represent mean ± SEM of n = 3 animals per group.
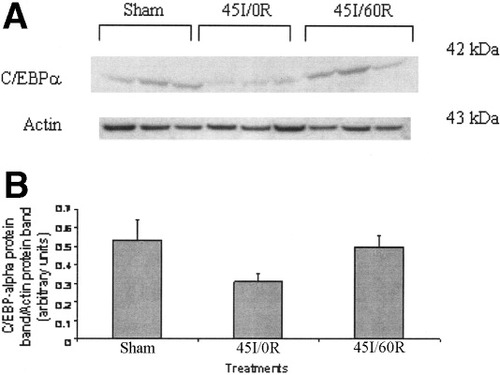
Western Blot analysis of liver C/EBPα after warm in vivo liver ischemia-reperfusion. Sham-control animals received saline, anesthetic, and laparotomy only, and livers of other groups were subjected 45 minutes of ischemia alone (45I/0R), or 45 minutes of ischemia plus 60 minutes of reperfusion (45I/60R), in vivo. Liver cytoplasmic fraction in Tris-EDTA (10 mmol/L Tris-HCl, 1 mmol/L EDTA, 0.1 mmol/L phenylmethol sulfonyl fluoride, and cØmplete protease inhibitor, pH 7.9) buffer, containing cytoplasmic C/EBPα protein was used for Western blot analysis. Protein (60 μg) was separated on NuPAGE gels (10%) and transferred to nitrocellulose membranes. Western blot analysis was done as described in Materials and Methods. Data represent mean ± SEM of n = 3 animals per group. (B) Densitometry of C/EBPα Western blot was performed as described in the legend to Figure 3. No statistically significant difference in liver C/EBPα was detected between groups.
Serum IL-6 Levels
Although serum IL-6 levels increased in both the 45I/0R and 45I/60R groups, the increase did not reach statistical significance (Fig. 7).
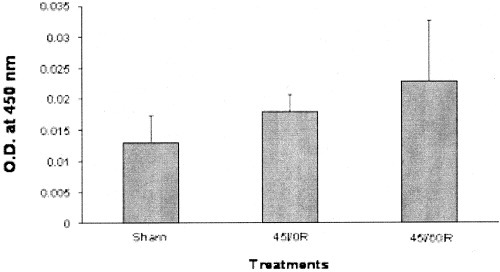
Serum IL-6 after warm in vivo liver ischemia-reperfusion. Sham-control animals received saline, anesthetic, and laparotomy only, and livers of other groups were subjected 45 minutes of ischemia alone (45I/0R), or 45 minutes of ischemia plus 60 minutes of reperfusion (45I/60R), in vivo. Blood samples were collected at the end of ischemia or reperfusion and serum IL-6 levels determined with a commercially available a kit according to the manufacturer's protocol. Data represent mean ± SEM of n = 3–4 animals per group. No statistically significant difference in serum IL-6 level was detected between groups.
Discussion
In this study, we investigated hepcidin expression under the pathophysiological condition of liver I/R injury. We report here the first evidence that modulation of hepcidin expression by liver ischemia alone, and by liver I/R. Under our experimental conditions, we were unable to detect hepcidin in serum from sham-control animals (Fig. 1). However, hepcidin has been reported to be constitutively expressed,5, 20, 21 and we were able to observe light immunohistochemical staining for hepcidin in liver sections from sham-control animals (Fig. 2). The reported secretion of hepcidin into the bloodstream and its eventual disposition in the urine, along with the staining pattern observed in this study, suggest a differential constitutive expression in hepatocytes of control animals, whereby hepatocytes proximal to liver venules appear to have the higher level of hepcidin expression. In the absence of iron overload and under conditions of acute inflammatory states, some blood-borne factor (tumor necrosis factor-alpha, IL-1, IL-6, or IL-12) probably elicits the induction of hepcidin in hepatocytes, followed by the subsequent reduction in blood iron observed with liver ischemia alone or liver I/R (Fig. 5).22
Unlike an earlier study, which reported that hypoxia suppressed hepcidin expression,6 we observed an increase in hepcidin mRNA levels with liver ischemia alone, and with liver ischemia-reperfusion. Differences in the experimental design of the current and earlier studies may explain the contrasting results. Nicolas et al. employed a hypobaric chamber to induce hypoxia in mice and measured hepcidin levels at much later time points of 1, 2, 4, and 12 days of hypoxia.6 Since our experimental condition of hypoxia is not exclusive of the systemic inflammatory component normally observed with ischemia or I/R of the liver, an alternative explanation is that the positive response of hepcidin expression by inflammation counterbalanced the negative effect on hepcidin levels reported with hypoxia.6, 23 However, our finding of increased hepcidin expression at 60 minutes of reperfusion is in agreement with the results Nicolas et al., who reported increased hepcidin levels with reoxygenation.6 Furthermore, with our model, we report the earliest reported induction of liver hepcidin, compared to the 4.3-fold induction in liver hepcidin mRNA at 90 minutes observed by Pigeon et al.1 in mice with systemic lipopolysaccharide treatment, and the 2-fold increase in liver hepcidin mRNA at 6 hours in a mouse turpentine-inflammatory model, reported by Nicolas et al.6 Hypoxia induces anemia, and in the presence of anemia, hepcidin expression is reduced.6, 7 It is unlikely in our model that hepcidin-mediated decrease in serum iron occurred due to erythropoetic demand or at the duodenal level, given the short duration of ischemia and reperfusion. Therefore, hepcidin may be working at the reticuloendothelial cell level (macrophage/KC). It has been reported that hypoxia also caused activation or increased RNA binding activity of iron regulatory proteins 1/2,24, 25, supposedly mediated by hypoxic inducible factor alpha. No structural elements required for the binding hypoxic factors have been reported in hepcidin transcripts.1, 6
Recently, using C/EBPα null mice, Courselaud et al. convincingly demonstrated that hepcidin expression is regulated via transcription factor C/EBPα.11 In this study, the decrease in cytoplasmic C/EBPα with liver ischemia alone and subsequent return to control levels by 60 minutes reperfusion suggest that translocation of C/EBPα might have occurred with liver ischemia, and that C/EBPα may modulate hepcidin expression as reported by Courselaud et al.11 The results obtained for C/EBPα in this study does not allow us to conclusively state that C/EBPα mediates liver hepcidin expression during liver I/R, since we were unable to detect nuclear C/EBPα under our experimental conditions. Furthermore, other transcription factors such as nuclear factor kappa B are known to be activated during liver I/R, and potential binding sites for the transcription factors hepatocyte nuclear factor-3 beta, C/EBPβ, and nuclear factor kappa B have been reported in the 5′ regulatory region of the hepcidin gene of mouse and humans. Therefore, the molecular mechanism responsible for the expression of hepcidin during liver I/R remains unclear.
In this study, we report the first evidence of the modulation of hepcidin expression by liver ischemia alone and liver I/R. Our results suggest that both ischemia and I/R modulate hepcidin expression in vivo. However, further studies are needed to identify the molecular mechanism of ischemia- and I/R-mediated hepcidin expression. This is particularly true in light of the results of this study showing that currently proposed modulators of hepcidin expression (IL-6, STAT-1, -3, and -5, and C/EBPα) do not appear to modulate hepcidin expression in our acute inflammatory I/R liver model.
Acknowledgements
The authors thank Dr. Saul Karpen (Department of Pediatrics, Section of Gastroenterology, Hepatology and Nutrition, Texas Children's Liver Center, Baylor College of Medicine, Houston, TX) for his perusal of the manuscript.