Optimal cycle of intermittent portal triad clamping during liver resection in the murine liver
Abstract
We designed this experimental study to determine the optimal cycle for intermittent inflow occlusion during liver resection. A cycle of intermittent clamping (IC) for 15 minutes of ischemia followed by reperfusion for 5 minutes during liver resection is currently the most popular protocol used by experienced liver centers. As each period of reperfusion is associated with bleeding, longer periods of clamping would be advantageous. However, the longest safe duration of successive ischemia is unknown. Three groups of mice were subjected to a total liver ischemic period for 90 minutes; 2 groups underwent IC for 15 or 30 minutes, respectively, followed by 5 minutes of reperfusion, while the control group was subjected to continuous inflow occlusion only. The degree of tissue injury was assessed using biochemical and histological markers, as well as animal survival. While serious injury was observed in the continuous clamping group, both IC groups were associated with minimal injury, including lesser degrees of apoptosis and necrosis. All animals survived in the IC groups, while all animals died following 90 minutes of continuous inflow occlusion. In conclusion, intermittent portal pedicle clamping with 15- or 30-minute cycles is highly protective. A period of 30 minutes clamping should be preferred, since this would decrease the amount of blood loss associated with each cycle. This data should be confirmed in humans, and may represent a change in the current practice of hepatic surgery. (Liver Transpl 2004;10:794–801.)
Intraoperative blood loss and the need for transfusion are major factors influencing morbidity and mortality following liver resection.1-3 Inflow occlusion (Pringle maneuver) associated with low central venous pressure is often used to prevent bleeding during transection of the hepatic parenchyma. However, this maneuver causes ischemia and reperfusion injury, with irreversible injury after about 60 to 75 minutes of ischemia.4-8 Two protective strategies have been proposed to enable longer periods of safe ischemia, namely intermittent clamping (IC) and ischemic preconditioning. Ischemic preconditioning, a short period of ischemia (10 minutes) and reperfusion (10–15 minutes) before a sustained ischemic insult, has been shown to be protective in several animal9-16 and human17, 18 studies. IC consists of successive cycles of 15 to 20 minutes of inflow occlusion separated by periods of 5 minutes of reperfusion.6, 7 We have shown in a mice model that ischemic preconditioning is comparable to IC for ischemic periods up to 75 minutes, but IC appears superior after longer periods of ischemia.8, 19, 20
Very long periods of ischemia have been reported using IC. For example, Sakamoto et al.21 reported good liver function after ischemic times of the liver exceeding 5 hours. However, IC has the inherent disadvantage of causing bleeding during each reperfusion phase.8 For example, a period of 90 minutes of ischemia requires 6 periods of reperfusion, for a total duration of 30 minutes. Thus, longer periods of ischemia for IC are suitable to decrease blood loss. Studies of different durations of ischemia for IC have provided controversial results.5, 22-24 In a rat model, IC of the liver for 15 or 30 minutes was associated with a comparable degree of injury as for periods of total ischemia of 60, 90, and 120 minutes.5 In contrast, another study in the rat demonstrated reduced injury with single-period clamping for 15 minutes when compared to longer periods such as 20 or 30 minutes.4
The purpose of this study was to test whether longer clamping periods (30 minutes) confer the same protection as shorter periods (15 minutes) for prolonged periods of total ischemia. Another aim of the study was to evaluate mechanisms of protection with a focus on the mechanisms of cell death.
Abbreviations
IC, intermittent clamping; TUNEL, terminal deoxynucleotidyl transferase d-uridine triphosphate nick end labeling; DNA, deoxyribonucleic acid.
Methods
Study Design
We studied 3 groups. The control group consisted of animals subjected to 90 minutes of inflow occlusion; the 2 experimental groups included respective cycles of IC of 15 or 30 minutes of ischemia, followed by 5 minutes of reperfusion, also for a total duration of 90 minutes of ischemia.
The degree of tissue injury was evaluated after 3 hours of reperfusion using a partial hepatic ischemia model.25 Biochemical and histological injury markers were used, including serum levels of hepatic enzyme, hematoxylin and eosin staining, and electron microscopy to detect apoptotic or necrotic cell death. Apoptosis was also investigated by terminal deoxynucleotidyl transferase d-uridine triphosphate nick end labeling (TUNEL) assay, caspase activity, cytochrome C release in the cytoplasm, and evidence of deoxyribonucleic acid (DNA) fragmentation. Finally, in a model of total vascular ischemia, we monitored animal survival for 30 days in each group.
Animals
Male wild-type mice (C57BL6) were used in all the experiments. The animals were fed a laboratory diet with water and food ad libitum until use and were kept under constant environmental conditions with a 12-hour light-dark cycle. All the procedures were performed in accordance with the Keimyung University Medical Science Institute Animal Care Committee guidelines.
Partial Hepatic Ischemia
Surgery was performed under isoflurane anesthesia (Choongwae-Abbott, Korea). Hepatic ischemia was achieved by the following method.26 The abdomen was entered through a midline incision and the liver was freed from its ligaments. The portal triad was dissected and a microvascular clamp (Aesculap, San Francisco, CA) was placed so that it produced ischemia of the median and left lobes, allowing intestinal blood flow through the right and caudate lobes. During the ischemic period, the abdomen was closed with a running suture of 4-0 chromic gut. The animal was kept under anesthesia during reperfusion, which was initiated by removing the clamp. The animal was allowed to recover from anesthesia during the 3 hours of reperfusion.
Serum Enzyme Analysis
Serum levels of aspartate aminotransaminase and alanine aminotransferase have been used as a general marker of hepatocyte injury.6, 8 Blood samples were obtained from the inferior vena cava after 3 hours of reperfusion. Blood cells were pelleted by immediate centrifugation at 10,000 g for 8 minutes at 4°C. Enzyme levels were measured using a serum multiple biochemical analyzer (COBAS Integra 800; Roche Diagnostic GmbH, Manheim, Germany).
Hematoxylin and Eosin Staining of Liver Tissue
A shorter period of ischemia (less than 60 minutes) and reperfusion results in apoptosis of sinusoidal endothelial cells, followed by apoptosis of hepatocytes, with minimal change in the intracellular organelles. A longer period (over 75 minutes) affects the parenchyma, with cellular swelling, vacuolization, and breakdown of cell organelles, resulting in necrosis.19 The morphologic changes in the liver were investigated to differentiate the degree of injury between the groups by light microscopy after hematoxylin and eosin staining.
Tunel Staining
Apoptosis leads to specific DNA strand breaks that can be labeled with modified nucleotides in an enzymatic reaction called the TUNEL assay.
The livers with peripheral tissues trimmed off, were fixed in vivo by perfusion with 4% paraformaldehyde (Sigma, St. Louis, MO) in phosphate-buffered saline administered through the portal vein. Frozen sections (5 μm) of the fixed tissue were prepared and stained by the TUNEL method using a commercial kit (Boehringer Mannheim Co., Indianapolis, IN). Morphometric analysis of the fluorescent cells was performed under high-power magnification (400×) in a blinded fashion. A total of 20 random sections were investigated per slide to determine the percentage of TUNEL-positive cells.
DNA Laddering to Observe DNA Fragmentation
Liver tissue was sonicated in DNA lysis buffer containing 5 mM (Tris), 20 mM EDTA, and 0.5% Triton® X-100 (all from Sigma, St. Louis, MO), and stored for 30 minutes in ice. The lysate was centrifuged and DNA was extracted using the phenol-chloroform method. The DNA was electrophoresed on 2% agarose gels, stained with ethidium bromide for 30 minutes, visualized with an ultraviolet transilluminator, and photographed with a Polaroid camera (DS34 Polaroid, United Kingdom).
Caspase 3 Activity
Caspase 3 activity was determined by measuring the proteolytic cleavage of the specific substrate N-acetyl-Asp-Glu-Val-Asp-pNA (Ac-DEVD-pNA; Calbiochem, Darmstadt, Germany) in the presence or absence of the specific inhibitor Z-Val-Ala-Asp(OMe)-CH2F (Z-VAD-FMK; Calbiochem, Darmstadt, Germany). Liver tissue was quickly excised and sonicated in assay buffer containing 50 mM Tris, 150 mM NaCl, 5 mM EDTA, 0.5% NP-40, 100 mM PMSF, 1 M DTT, 10 μg leupeptin, 10 μg aprotinin (all from Sigma, St. Louis, MO). The protein content was determined using the Bradford protein assay (BioRad, Hercules, CA). The samples were incubated at 37°C with Ac-DEVD-pNA substrate in the presence or absence of the inhibitor Z-VAD-FMK. The caspase 3 activity was measured by the release of p-nitroaniline from Ac-DEVD-pNA substrate at 405 nm at the indicated time-points. p-Nitroaniline release was measured using a Techan Sunrise enzyme linked immunosorbent assay reader (San Jose, CA)
Cytochrome C Release
The cells were homogenized in 10 volume/weight buffer A (20 mM HEPES-KOH (pH 7.4), 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 0.1 mM PMSF, 250 mM sucrose (all from Sigma, St. Louis, MO) using a Dounce grinder (Kontess Glass Co., Vineland, NJ). Unbroken cells and nuclei were pelleted at 400 g for 5 minutes at 4°C. The supernatant was further centrifuged at 12,000 g for 10 minutes to pellet the mitochondria. The supernatant was analyzed using the Bradford protein assay (BioRad, Hercules, CA), boiled with 3× SDS sample buffer for 90 seconds, and subjected to 12% SDS-PAGE (Novex, San Diego, CA). The proteins were transferred onto a nitrocellulose filter and probed with mouse monoclonal anti-cytochrome C antibody (BD Biosciences, San Diego, CA), followed by a secondary antibody conjugated to horseradish peroxidase and detected with enhanced chemiluminescence detection reagents (Amersham, Piscataway, NJ).
Morphologic Examination by Electron Microscopy
For transmission electron microscopy, specimens were fixed in 2.5% glutaraldehyde in 0.1 mol/L phosphate buffer at pH 7.4, rinsed for 30 minutes in 3 changes of 0.1 mol/L phosphate buffer at pH 7.2, postfixed for 2 hours in 1% osmium tetroxide, dehydrated in graded alcohol solutions, washed in 100% propylene oxide, and embedded in Epon (Polysciences, Warrington, PA). Sections of 1 micron each were stained by 1% toluidine blue, and areas with centrilobular, periportal hepatocytes, and endothelial cells were selected. Thin sections (40–60 nm) were cut on a microtome (Sorvall MT-5000; DuPont, Newtown, CT) and placed on 200-mesh copper grids. Ultrathin sections were stained with uranyl acetate and lead citrate. Photomicrographs were taken on a Hitachi-600 electron microscope operating at 80 kV.
Animal Survival After Total Hepatic Ischemia
Animal survival was evaluated using a modification of the total hepatic ischemia model.25 Briefly, after applying ischemia using the same time protocols, the abdomen was reopened and the microvascular clamp was removed. The right and caudate lobes were then resected, leaving only ischemic liver in place. The abdomen was closed and the animals were allowed to recover from anesthesia and closely monitored for 30 days.
Statistical Analysis
The results are expressed as means ± standard error. Data were analyzed using SPSS software version 10.0.0 (SPSS Inc., Chicago, IL). The differences between the groups were evaluated using Student's t-test. Animal survival was compared using Fisher's exact test. P < .05 was considered statistically significant.
Results
Does IC With 15- and 30- Minute Cycle Confer the Same Degree of Protection Against Reperfusion Injury?
Serum transaminase, an established marker of hepatocyte injury, was used to assess reperfusion injury.27 Serum levels of these enzymes were significantly lower in both IC groups when compared to the continuous clamping group (P < .05). There was no difference between the 15- and 30-minute intermittent occlusion groups (Fig. 1). Reperfusion injury was also assessed in hematoxylin and eosin stained biopsy taken 3 hours after reperfusion. The histologic appearance of the hepatic parenchyma after ischemic challenge with 15- and 30-minute cycles for a total of 90 minutes showed little alteration of parenchyma or derangement of the hepatic architecture, and no differences between the 2 groups. In contrast, the control group with continuous ischemia for 90 minutes showed severe alteration of parenchyma, such as cellular swelling, blood congestion in the sinusoids, cellular lysis, and cytoplasmic vacuolization with inflammatory cell infiltration (Fig. 2).
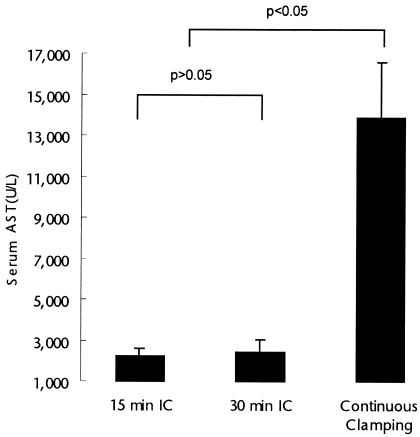
Serum aspartate aminotransaminase (AST) levels, used as a marker of hepatocyte injury, were significantly lower in both intermittent clamping (IC) groups, when compared to continuous inflow occlusion. The total duration of ischemia was 90 minutes in each group. There was no significant difference between both IC groups (analysis of variance (ANOVA); P < .05).
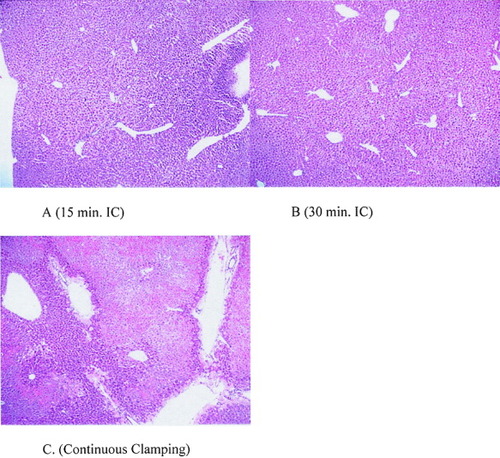
Tissue sections stained with hematoxylin and eosin (H&E; 40×). The hepatic parenchyma of both intermittent clamping groups (A, B) were well preserved. By contrast, severe parenchymal alterations including severe geographic necrosis and marked venous dilatation were seen in the continuous ischemia group (C). The total duration of ischemia was 90 minutes in each group. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Does IC Prevent Apoptotic Cell Death or Necrosis?
Apoptosis and necrosis of hepatocytes are key features of cell death after reperfusion of a warm ischemic liver.13, 28-30 We evaluated several markers of apoptotic cell death, including DNA laddering, TUNEL assay, caspase activity, and cytochrome C release at 3 hours of reperfusion after the respective ischemic challenges. We used the TUNEL assay to quantify the number of apoptotic hepatocytes, and DNA laddering by electrophoresis to detect DNA fragmentation. Electron microscopy was also used to assess the type of cell injury. There were few TUNEL-positive hepatocytes in both IC groups, and no difference between the 2 groups. In contrast, the number of TUNEL-positive hepatocytes was significantly higher in the control group (Fig. 3). Similarly, DNA fragmentation was not identified in both IC groups, while typical laddering was noted in the control group (Fig. 4). Both caspase 3 activity and cytochrome C release from mitochondria into the cytoplasm were also significantly lower in the 2 intermittent groups than in the control group, and again there was no difference between the 2 IC groups (Figs. 5 and 6). Electron microscopy allows a fine assessment of the morphologic features associated with apoptotic cell death after warm ischemia and reperfusion injury of the liver.29, 31 There was no evidence of apoptosis in either IC group. The microvilli on the sinusoidal surface of the hepatocytes were normal in shape; cytoplasmic processes of the sinusoidal endothelial cells lined the sinusoidal space, and collagen deposits were also seen in Disse's space (Fig. 7). Following 90 minutes of continuous ischemia, detachment of sinusoidal endothelial cells with loss of the cytoplasmic processes lining the sinusoid was seen, and the sinusoidal endothelial cells showed rounding and margination of chromatin and apoptotic bodies in the cytoplasm. In many areas, Kupffer cells contained apoptotic bodies.
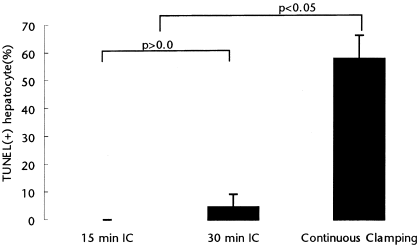
TUNEL staining was used as a marker of apoptotic cell death. Both intermittent clamping (IC) groups show significantly low positive cells when compared to the continuous group. There was no significant difference between both IC groups (analysis of variance (ANOVA); P < .05).
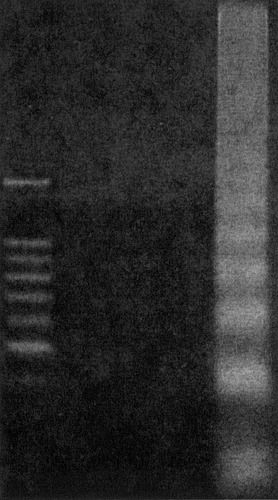
Agarose gel electrophoresis of deoxyribonucleic acid (DNA) from livers after reperfusion for 3 hours with varying ischemic insults showed no DNA laddering in intermittent clamping (IC). By contrast, the livers subjected to continuous ischemia showed the typical laddering pattern, indicating DNA fragmentation.
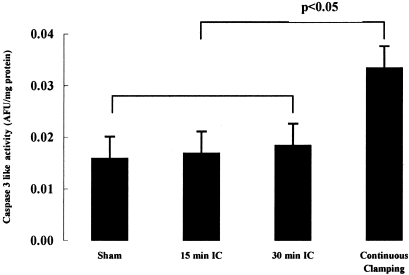
Caspase 3 activity measured in hepatic tissues was significantly lower in the 15- and 30-minute intermittent clamping (IC) groups than in the continuous group (P = .004); there was no difference between the IC groups (P = .1). The sham operated group showed low values, similar to the 2 IC groups.
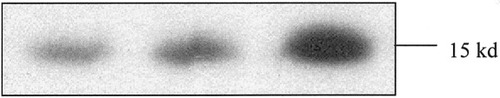
Cytochrome C released from mitochondria detected by Western blot analysis was lower in both intermittent clamping (IC) groups (15- and 30-minute IC) than in the group with 90 minutes of continuous ischemia.
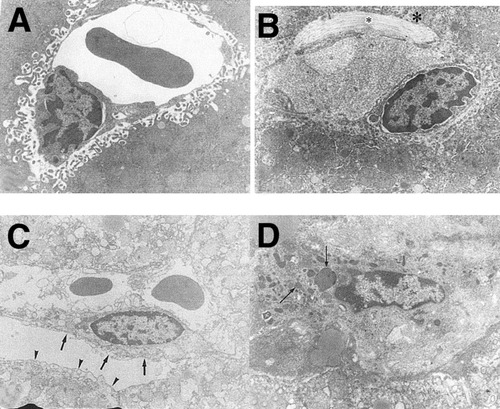
Representative electron micrographs. In the liver with 15- and 30-minute intermittent clamping (IC) cycle, sinusoidal endothelial cells and microvilli of hepatocytes were intact, with collagen deposit (*). A: 15-minute IC, 7,000×. B: 30-minute IC, 8,000×. In the liver with continuous 90-minute ischemia, detachment of sinusoidal endothelial cells (short arrows), loss of microvilli (arrow head), margination of chromatin, and many apoptotic bodies (long arrows) were detected. C: 7,000× D: 10,000×.
Does IC Protect Against Animal Death Following Prolonged Period of Ischemia?
In order to establish the relevance of the protective effects of IC, we conducted an animal survival study using a total hepatic ischemia model,25 after applying the same 3 ischemia/reperfusion treatments as used in the biochemical and histologic study. All 5 animals in each group treated with IC (15- or 30-minute cycles for a total of 90 minutes) survived for 30 days, while all the animals in the continuous ischemia group died within three days (P = .04).
Discussion
While prolonged inflow occlusion is often used during liver surgery to prevent bleeding, the best way to apply this strategy remains unknown. IC of the portal triad remains the safest modality for prolonged periods of ischemia beyond 90 minutes.19 In this study, we demonstrate that IC using longer cycles of ischemia (30 minutes) than usually applied (15 minutes) is safe, and confers similar degree of protection. Regarding the time-point studied, we choose 30 minutes because this is the longest ischemic time associated with minimal detectable injury in this model and in the human situation.4, 5, 17, 18 Studying longer periods of ischemia could be performed, but will likewise be associated with significant injury. The finding that repeated 30-minute ischemia interrupted by 5-minute reperfusion can be safely applied is quite provocative, and may have significant clinical impact. This strategy, applied to the human situation, would result in reduced bleeding due to the need for fewer reperfusion periods during liver resection for cancer or transplantation. The study also indicates that IC with short or long cycles confers protection through similar mechanisms as ischemic preconditioning, with a primary effect of preventing apoptosis and, possibly, subsequent necrosis.
The first aim of the study was to test whether longer ischemic periods for IC are safe. The literature provides contradictory results in animal models,4 and no clinical data is available. A clinical controlled study by Belghiti et al.8 demonstrated superior protection of IC with cycles of 15 minutes followed by 5 minutes of reperfusion than with continuous clamping, in patients undergoing hepatic surgery. The increased tolerance of parenchyma to IC over continuous clamping was noted in the patient with normal, and especially with abnormal, liver parenchyma.8 Another clinical study by Horiuchi et al.4 suggested decreased tolerance of the liver for ischemic cycles exceeding 15 minutes. However, in this study, only 1 time point after reperfusion was evaluated. Animal models in rats have provided contradictory results.5, 22-24 Our study provides strong evidence that there is no difference between 15- and 30-minute IC cycles in protecting against both apoptosis and necrosis. The demonstration in this study of similar protection with periods of 15 and 30 minutes of ischemia is important, as longer ischemia will enable to have less blood loss during the reduced number of reperfusion periods.
Studies in various animal models31, 32 and human19 have shown that apoptosis of sinusoidal endothelial cells followed by apoptosis and necrosis of hepatocytes are key features of normothermic hepatic ischemia. Protective strategies such as ischemic preconditioning and IC have also been shown to inhibit the apoptotic cascade.13, 19 Others have suggested that the mechanism of cell death following normothermic ischemia is instead oncotic necrosis.33 The pathway of reperfusion injury has been an interesting debate over the past few years, as both features of apoptosis or necrosis are typically present after reperfusion of the ischemic liver, and the term “necrapoptosis” has been proposed by Lemasters et al.34, 35 to emphasize the shared pathways leading to both forms of cell death. The results of this study support the concept of necrapoptosis as a central feature of normothermic ischemia and reperfusion injury of the liver. Severe necrosis of hepatocytes with evidence of activation of the apoptotic pathway was consistently documented following continuous ischemic insult for 90 minutes. Apoptosis of sinusoidal endothelial cells was an early feature of injury, as has previously been shown.30-32, 36
Both IC protocols disclosed strong protection against apoptosis and necrosis, including complete absence of derangement in the architecture of the liver. The large number of endpoints used to assess necrosis (hematoxylin and eosin staining, electron microscopy) and apoptosis (TUNEL staining, DNA laddering, cytoplasmic caspase 3 activity, cytochrome C release, and electron microscopy) represent convincing evidence related to the mechanisms of injury. Caspase 3–like activity has been measured in the sham operated group and shows low values similar to those in the 2 IC groups. Reportedly, there are significant increases in caspase activities after reperfusion and the protective effects of caspase inhibitors in several models of hepatic ischemia.36-38 Of interest, these results are also consistent with previous data comparing ischemic preconditioning with IC, in which protection in terms of animal survival was associated with decrease of apoptotic activity and absence of necrosis on hematoxylin and eosin stained biopsies.19 These data may highlight that effective protection against ischemic injury should target prevention of apoptosis, which likewise will also prevent the development of subsequent necrosis.
The relevance of histologic and biochemical markers in animal models of hepatic ischemia needs to be confirmed by experiments looking at animal survival. We found complete protection of both IC protocols in terms of animal survival following prolonged periods of total hepatic ischemia, while an equivalent duration of ischemia provided in a continuous manner consistently caused animal death within 72 hours after surgery. These results established the relevance of IC in protecting the liver against long periods of ischemia.
The current model is a standard model, which has been used in many other ischemia/reperfusion studies. Additionally, it has been shown that the murine model of hepatic injury often better mimics the clinical situation than rat or even larger animal models.39 Similar tolerance to ischemia and common pathways of injury have been demonstrated in the mouse and human liver.13, 17 It is not known whether repeated 30-minute cycles of ischemia can be safely applied clinically, and experiments in a validated model are necessary. Therefore, we would attest that these results are sufficient to conduct a safe human study in patients undergoing liver resection to identify the best IC strategy. Of note, some surgeons have reported safe use of long cycles (20–30 minutes) of ischemia during liver resection performed under intermittent vascular inflow occlusion for more than 2 hours (M. Makuuchi, personal communication).
In conclusion, IC confers superior protection to continuous clamping for prolonged periods of hepatic ischemia. The optimal cycle of IC is important to reduce bleeding and hepatocellular injury in a clinical setting. Our results indicated that intermittent portal pedicle clamping with 30-minute cycles is highly protective, and should be preferred to shorter periods to enable optimal protection with decreased blood loss associated with each cycle. These data should be confirmed in a human trial, and might represent a change in the current practice of hepatic surgery.