Heme oxygenase-1 potentiates the survival of small-for-size liver graft
Telephone: 852-28199653; FAX: 852-28199643
Abstract
This study aims to clarify the role of heme oxygenase-1 (HO-1) in small-for-size liver transplantation. Transplantation was performed using 40% small-for-size or 100% whole liver grafts in rats. When no treatment was given, over-expression of HO-1 was detected predominantly in the small-for-size grafts at 6 hours after reperfusion as compared to whole grafts in both syngeneic and allogeneic combinations. Recombinant adenoviral vector encoding HO-1 gene (AdHO-1) administered to donors 48 hours before transplantation enhanced HO-1 expression in both whole and small-for-size allografts, with a predominant augmentation in the small-for-size allografts, suggesting favorable conditions for the induction of HO-1 expression in small-for-size allografts. In close relation to the expression level of HO-1, AdHO-1 significantly prolonged both whole and small-for size allograft survivals, with a remarkable effect in the small-for-size allograft group. The prolongation of allograft survival was blocked by the HO-1 inhibitor (zinc protoprophyrin IX). The non-treated small-for-size allografts demonstrated impaired liver function during the early period after reperfusion, which could be improved by over-expression of HO-1, but reversed by the HO-1 inhibitor. The markedly increase expression HO-1 in small-for-size allografts was associated with lower levels of adhesion molecules and pro-inflammatory cytokines in the early phase after reperfusion. These findings support the beneficial effects of HO-1 on allograft survival. In conclusion, the ability of small-for-size grafts in the induction of HO-1 expression might facilitate their own survival in liver transplantation. (Liver Transpl 2004;10:784–793.)
Partial grafts from split livers or live donors have become a valuable alternative in solving the problem of organ shortage.1, 2 There are, however, more risks in performing partial or small-for-size liver transplantation, not only due to technical obstacles, but also early graft loss after microcirculatory damage.3-5 In addition, the microcirculatory and cellular injuries in small-for-size allografts might trigger profound immune responses.6 However, the molecular basis that leads to early graft loss and whether induction of stress proteins during the early period after reperfusion can benefit small-for-size grafts remain to be determined.
Heme oxygenase-1 (HO-1) is an inducible protein in response to various stimuli associated with oxidative stress.7 It cleaves pro-oxidant heme into equimolar amounts of carbon monoxide, free iron, and biliverdin, which is converted to bilirubin by biliverdin reductase.8 Although expression of HO-1 in normal liver is restricted to a subpopulation of Kupffer cells, the gene is expressed in both parenchymal and particularly non-parenchymal liver cells under stress conditions.9, 10 “Protective effects” of enhanced HO activity, probably through production of carbon monoxide, have been reported in various disease-related models.11-14 However, the functional role of HO-1 in the context of hepatocellular injury is still controversial. A massive amount of HO-1 might even lead to cytotoxicity by increasing the susceptibility to oxidative stress rather than cytoprotection.15 It seems that the therapeutic potential of HO-1 may be limited to a narrow window.
In the situation of small-for-size liver transplantation, the role of HO-1 is not clear. At the time of reperfusion, excessive blood inflow (in relation to the graft size)16 carries more oxygen to the cells in small-for-size grafts, subsequently generating more reactive oxygen species. This increases the susceptibility of liver cells to the apoptotic stimuli and the mechanical injury associated with transient portal hypertension in small-for-size grafts. As a member of the stress protein family, the expression of HO-1 may reflect the severity of small-for-size graft damage. However, early HO-1 expression may also confer a higher basal level of cytoprotection, which, in turn, affects the liver graft function and survival. In the present study, we compared the expression patterns of endogenous HO-1 in small-for-size and whole liver grafts in both syngeneic and allogeneic settings, and introduced exogenous HO-1 to the allografts to investigate whether this approach could achieve any beneficial effects in graft survival.
Abbreviations
HO, heme oxygenase; AdHO-1, adenoviral vector encoding HO-1 gene; AdLacZ, adenoviral vector encoding LacZ gene.
Materials and Methods
Preparation of Adenoviral Vectors
Rat HO-1 gene was cloned as previously described.14 The subcloning of rat HO-1 gene or reporter gene LacZ to vector plasmid of recombinant adenovirus and the production of adenoviral vector encoding HO-1 gene (AdHO-1) and adenoviral vector encoding LacZ gene (AdLacZ) were performed according to the manufacturer's instruction (AdMAX system; Microbix, Toronto, Canada). The large scale of adenoviral vectors were then purified using an Adenovirus Viraprep Kit (Virapur, Carlsbad, CA).
Animals and Surgical Procedures
DA (RT1a) and LEW (RT1l) rats were originally purchased from the Animal Resources Centre (Murdoch, Australia) and maintained in the Laboratory Animal Unit, the University of Hong Kong. All experimental procedures were performed according to the institutional guidelines and approved by the Committee on the Use of Live Animals for Teaching and Research. Orthotopic liver transplantations were performed using DA and LEW rats weighing from 250–300 gm as donors and recipients, respectively. Liver grafts of 100% (whole) or 40% (small-for-size) of the original weight of recipients' livers (graft size was reduced by lobe ligation) were used for implantation using the cuff's technique as described.17 Experimental groups included: 1) whole isograft (LEW to LEW), no treatment; 2) 40% isograft, no treatment; 3) 40% isograft with AdHO-1 5 × 1010 pfu intravenous injection to the donor 48 hours before transplantation; 4) whole allograft (DA to LEW), no treatment; 5) 40% allograft, no treatment; 6) whole allograft with AdLacZ 5 × 1010 pfu intravenous injection to the donor 48 hours before transplantation; 7) 40% allograft with AdLacZ; 8) whole allograft with AdHO-1; 9) 40% allograft with AdHO-1; 10) 40% allograft with AdHO-1 and zinc protoprophyrin IX (HO-1 inhibitor; Prophyrin Products, Logan, UT) 5 mg/kg/day intraperitoneal injection daily after transplantation. Death of recipients was defined as complete rejection and was confirmed by histopathology.
Liver Biochemistry, Immunohistochemistry, Reverse Transcriptase-Polymerase Chain Reaction, and Western Blot Analysis
Liver grafts were harvested at 6, 24, 48, and 72 hours after transplantation. Tissue samples were snap frozen and stored at −70°C. Plasma samples were collected for liver biochemistry examination. Hematoxylin and eosin staining was used to evaluate the histology of the grafts at various time points. The phenotype of graft infiltrating cells was detected by immunohistochemistry using the horseradish peroxidase protocol. Mouse anti-rat macrophage (ED1; Serotec, Oxford, UK) and HO-1 (OSA-111; Stressgene, Palo Alto, CA) monoclonal antibodies were used to detect graft infiltrating cells and HO-1 expression. Total ribonucleic acid was extracted from the snap-frozen tissue using the RNeasy Mini Kit (Qiagen, Valencia, CA). Reverse transcriptase polymerase chain reaction was performed to detect HO-1 messenger ribonucleic acid levels in the allografts. The protein level of HO-1 was determined by standard Western blot analysis using 12% sodium dodecylsulfate-polyacrylamide gel electrophoresis gel.
Measurement of HO Enzymatic Activity
HO enzymatic activity was measured by 2 of its end products, carbon monoxide and biliverdin/bilirubin. The amount of carbon monoxide was measured by the determination of carboxyhemoglobin in the peripheral blood of animals using spectrophotometry as described.18 The level of generated bilirubin in liver samples was measured by the method as follows. In brief, frozen samples of liver grafts were homogenized in ice-cold sucrose and Tris-HCl buffer. The microsomal pellet was obtained after centrifugation and re-suspended in MgCl2-potassium phosphate buffer. Sample protein was then incubated with the reaction mixture containing rat liver cytosol, hemin, glucose-6-phosphate, glucose-6-phosphate dehydrogenase, and nicotinamide adenine dinucleotide phosphate (Sigma-Aldrich, St. Louis, MO) for 60 minutes at 37°C. Generated bilirubin was measured by reacting with diazotized surfanilic acid to yield azobilirubin using spectrophotometry. The level of intragraft bilirubin was described by a ratio of sample/normal liver.
Statistical Analysis
Survival rate data were evaluated by the Kaplan-Meier method and compared by log-rank test. Data on the rest of the experiments were analyzed using Student's t-test. P < .05 was considered statistically significant.
Results
Small-For-Size Liver Grafts Highly Expressed HO-1 During the Early Phase After Transplantation
To determine the role of HO-1 in the context of small-for-size liver transplantation, expression patterns of HO-1 in small-for-size and whole liver grafts were compared by reverse transcriptase polymerase chain reaction and Western blot at various time points after transplantation. HO-1 was highly expressed at both messenger ribonucleic acid and protein levels in both small-for-size isografts and allografts, particularly at 6 hours after transplantation (n = 3, Figs. 1 and 2). AdHO-1 gene transfer remarkably enhanced the expression of HO-1, predominantly in the small-for-size allograft group (n = 3, Fig. 2). In addition, the expression of HO-1 in allografts was also confirmed by immunohistochemical staining (n = 3, Fig. 3). The generation of microsomal bilirubin and production of carboxyhemoglobin in peripheral blood corresponded to HO-1 protein expression (Fig. 4).
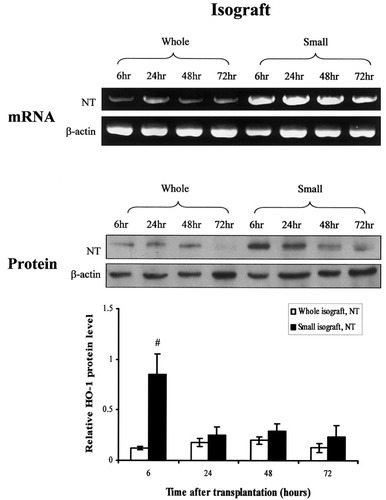
Detection of heme oxygenase-1 (HO-1) messenger ribonucleic acid (mRNA) and protein levels in isografts by reverse transcriptase-polymerase chain reaction and Western blot. Higher levels of HO-1 mRNA and protein were determined in the small-for-size isografts at 6 hours after reperfusion. NT, no treatment; #P< .05, compared with whole isografts, NT.
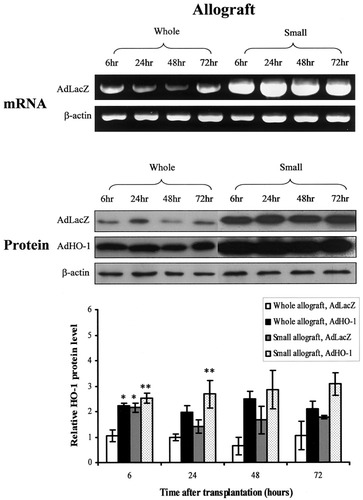
Determination of heme oxygenase-1 (HO-1) messenger ribonucleic acid (mRNA) and protein levels in allografts during the early post-transplant phase. Representative picture of reverse transcriptase-polymerase chain reaction and Western blot showed an increase of HO-1 expression in both mRNA and protein levels in the small-for-size allografts as early as 6 hours after liver transplantation. Adenoviral vector encoding HO-1 gene (AdHO-1) administration enhanced HO-1 expression in both whole and small-for-size allografts. Data were shown as mean ±sem after normalization with β-actin, and compared by Student's ttest. * P< .05, compared with whole allografts, adenoviral vector encoding LacZ gene (AdLacZ). ** P< .05, compared with small allografts, AdLacZ.
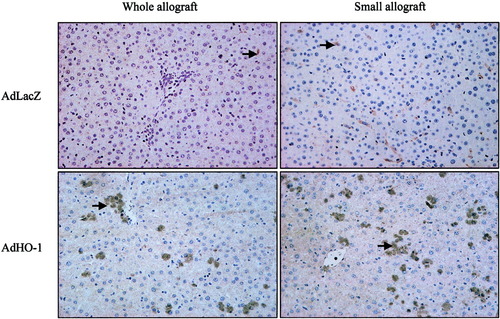
Immunohistochemical staining to detect the expression of heme oxygenase-1 (HO-1) in allografts. The number of HO-1 positive (HO-1+) cells was significantly increased in both whole and small-for-size allografts after adenoviral vector encoding HO-1 gene (AdHO-1) transduction (48 hours after AdHO-1 delivery). Arrows pointed to the HO-1+ cells. Magnification, ×200. AdLacZ, adenoviral vector encoding LacZ gene.
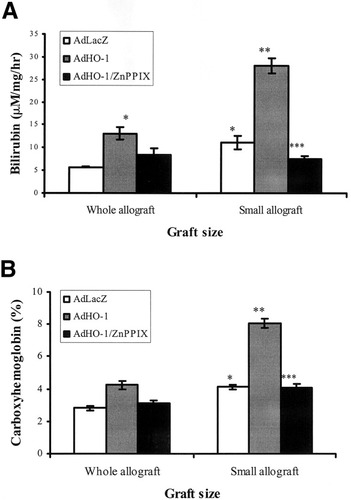
A representative diagram of HO enzymatic activity by measurement of (A) generated bilirubin from microsomal protein of the liver sample and (B) carboxyhemoglobin in the peripheral blood of recipients at 6 hours after transplantation. The adenoviral vector encoding LacZ gene (AdLacZ)-treated small-for-size allografts presented a significantly higher level of bilirubin generation in microsome and carboxyhemoglobin in peripheral blood than AdLacZ-treated whole allografts. Adenoviral vector encoding HO-1 gene (AdHO-1) further enhanced the enzyme activity in small-for-size allografts, whereas the enhancement was blocked by zinc protoporphyrin IX (ZnPPIX). * P< .05, compared with whole allografts with AdLacZ; ** P< .05, compared with small allografts with AdLacZ; *** P< .05, compared with small allografts with AdHO-1. Data were representative of 3 animals in each group.
Liver Function Was Improved by Over-Expression of HO-1 in Small-For-Size Allografts During Early Period After Transplantation
An elevation of plasma alanine aminotransferase level was detected in the small-for-size allograft group at all time points, particularly at 6 hours after reperfusion, compared with the whole allografts. Introduction of AdHO-1 greatly down-regulated the plasma alanine aminotransferase level in the small-for-size allograft group, whereas the down-regulation effect was blocked by zinc protoporphyrin IX administration (Fig. 5).
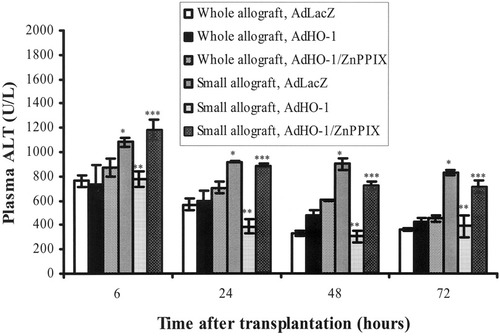
Liver biochemistry of the whole and small-for-size allografts during the early period after transplantation. The adenoviral vector encoding LacZ gene (AdLacZ)-treated small-for-size allografts demonstrated higher levels of alanine amontransferase (ALT) than AdLacZ-treated whole allografts at all the observed time points. Introduction of adenoviral vector encoding HO-1 gene (AdHO-1) to the small-for-size allografts remarkably decreased the ALT levels, whereas the reductive effect of AdHO-1 was blocked when zinc protoporphyrin IX (ZnPPIX) was administered. * P< .05, compared with whole allografts with AdLacZ; ** P< .05, compared with small allografts with AdLacZ; *** P< .05, compared with small allografts with AdHO-1. Data were demonstrated as mean ±sem, and compared by Student's t test.
The Effects of HO-1 on Allograft Survival Were Associated With Graft Size
To further examine the functional role of HO-1 in small-for-size liver transplantation, survival rates of small-for-size and whole liver grafts, with or without HO-1 over-expression, were compared in both syngenic and allogenic combinations. It was found that both small-for-size isografts and allografts survived shorter than whole isografts and allografts when no treatment or AdLacZ was given, respectively. Administration of AdHO-1 to small-for-size isografts demonstrated an improvement trend, whereas a remarkable prolongation of survival time was observed in small-for-size allografts (median survival time: 22 days with AdHO-1 vs. 6 days without AdHO-1, P < .01), and the survival time was shortened to 6 days when AdHO-1 was administered with zinc protoporphyrin IX (P < .01). AdHO-1 administration could also improve whole allograft survival (median survival time: 12 days with AdHO-1 vs. 8 days without AdHO-1). Transduction of LacZ gene by adenoviral vector did not influence both whole and small-for-size allograft survivals (Table 1).
| Groups | Graft Size | Number | Treatment | Survival (Days) | P Value |
|---|---|---|---|---|---|
| 1 | Whole isograft | 5 | NT | >100, >100, >100, >100, >100 | |
| 2 | Small isograft | 7 | NT | 4, 5, 5, >100, >100, >100, >100 | |
| 3 | Small isograft | 6 | AdHO-1 | 5, >100, >100, >100, >100, >100 | |
| 4 | Whole allograft | 7 | NT | 7, 7, 8, 8, 9, 9, 9 | |
| 5 | Small allograft | 7 | NT | 3, 5, 6, 6, 7, 7, 8 | <0.01* |
| 6 | Whole allograft | 5 | AdLacZ | 7, 8, 9, 9, 9 | |
| 7 | Small allograft | 5 | AdLacZ | 5, 6, 6, 8, 8 | <0.05† |
| 8 | Whole allograft | 6 | AdHO-1 | 10, 10, 12, 12, 13, 14 | <0.01† |
| 9 | Small allograft | 7 | AdHO-1 | 5, 14, 21, 22, 22, 24, 26 | <0.01‡ |
| 10 | Small allograft | 5 | AdHO-1+ZnPPIX | 4, 5, 6, 6, 7 | <0.01§ |
- Abbreviations: NT, no treatment; AdHO-1, adenovirus vector encoding heme oxygenase 1 gene, 5 × 1010 pfu intravenous injection to donor 48 hours before transplantation; AdLacZ, adenovirus vector encoding LacZ gene, 5 × 1010 pfu intravenous injection to donor 48 hours before transplantation.
- * Compared with group 4.
- † Compared with group 6.
- ‡ Compared with group 7.
- § Compared with group 9.
HO-1 Suppressed Early Inflammatory Responses After Liver Transplantation
To understand the mechanism that might involve the beneficial effects of HO-1 on graft survival in small-for-size liver transplantation, various parameters associated with early inflammatory responses after transplantation were determined. A larger number of infiltrating cells were detected in the periportal area of the small-for-size allografts than in whole allografts at 48 hours after reperfusion (Fig. 6A), and the majority of these cells were macrophages. AdHO-1 transduction of whole and small-for-size allografts presented a decreased number of macrophages, particularly in the small-for-size allograft group (Fig. 6B). Pro-inflammatory cytokines, tumor necrosis factor-alpha, and interleukin-1 beta were highly expressed in the small-for-size allografts earlier than the whole allografts at 6 hours after reperfusion. HO-1 over-expression could obviously reduce the levels of tumor necrosis factor-alpha and interleukin-1 beta, especially their expression in small-for-size allografts at 6 and 24 hours after reperfusion. The expression pattern of adhesion molecules, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 in whole and small-for-size allografts demonstrated a similar pattern as pro-inflammatory cytokines: early up-regulation in small-for-size allografts and inhibition by AdHO-1. In contrast, administration of AdHO-1 did not affect the early (6 and 24 hours after reperfusion) protein expression of pro-inflammatory cytokines and adhesion molecules in whole allografts (Fig. 7).
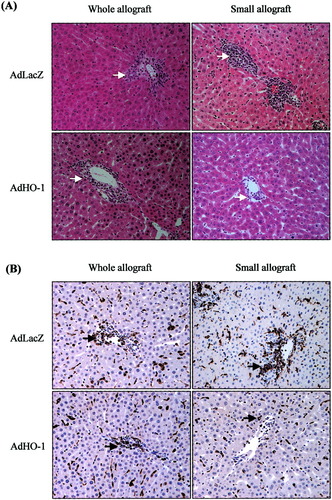
Histology of whole and small-for-size allografts at 48 hours after reperfusion. (A) An increased number of infiltrating cells in the peri-portal area were detected in the adenoviral vector encoding LacZ gene (AdLacZ)-treated small-for-size allograft compared with whole allograft, whereas adenoviral vector encoding HO-1 gene (AdHO-1) administration reduced the number of infiltrating cells in both whole and small-for-size allografts, particularly in the small-for-size allograft. Arrows pointed to the cells located in the peri-portal area. (B) By immunohistochemical staining, the majority of cells that located in the peri-portal area of allografts were found to express macrophage marker (ED1). AdHO-1 pretreatment obviously decreased the number of macrophages in both whole and small-for-size allografts, especially in the small-for-size allografts. Arrows pointed to the ED1 positive cells. Magnification, ×200.
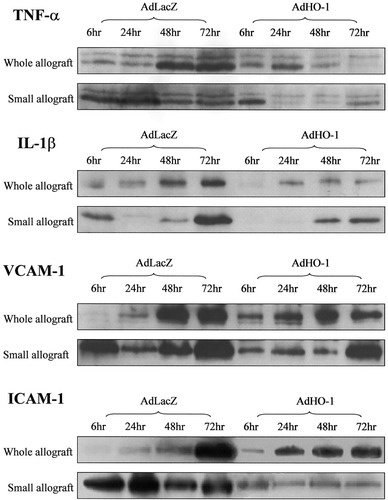
Detection of pro-inflammatory cytokines and adhesion molecules by Western blot. Up-regulation of tumor necrosis factor-alpha (tumor necrosis factor-alpha), interleukin-1 beta (interleukin-1 beta), vascular cell adhesion molecule-1 (VCAM-1), and intercellular adhesion molecule-1 (ICAM-1) was detected in small-for-size allografts as early as 6 hours after reperfusion, whereas an obvious elevation of these molecules in whole allografts was determined at 48 and 72 hours. The approach of adenoviral vector encoding HO-1 gene (AdHO-1) dramatically down-regulated the levels of tumor necrosis factor-alpha, interleukin-1 beta, VCAM-1, and ICAM-1, especially at 6 and 24 hours after reperfusion, whereas its effects on whole allografts were more predominant at 48 and 72 hours. AdLacZ, adenoviral vector encoding LacZ gene.
Discussion
In the present study, we demonstrated that small-for-size allografts displayed impaired liver function and damaged liver structure (data not shown) during the early period after reperfusion. At the same time, over-expression of HO-1 was also detected in these isografts and in the parallel allografts, indicating that the expression pattern of HO-1 might reflect the injury processes in the small-for-size liver grafts. However, it was not clear whether the endogenous elevation of HO-1 was a consequence of the damage or whether itself was a cause of the injury. Interestingly, some other factors, such as the “status” of grafts, might also affect the expression of HO-1, as some of our previous studies revealed a lower HO-1 messenger ribonucleic acid level in the “losing” small-for-size isografts.19 However, when the gene transfer approach induced over-expression of HO-1 in the small-for-size allografts, the survival time was prolonged and was accompanied with a reduced number of infiltrating cells, down-regulation of adhesion molecules, and improvement of liver function. This suggests that the increase of endogenous level of HO-1 in the small-for-size allografts might be an essential feedback to minimize liver injury. The fact that the inhibition of HO activity by zinc protoporphyrin IX could reverse the effects of AdHO-1 further revealed the protective role of this molecule in small-for-size allografts.
The overall effects of HO-1 on the prolongation of allograft survival could be reflected by the improvement of liver function and a decreased number of infiltrating mononuclear cells, down-regulation of pro-inflammatory cytokines (tumor necrosis factor-alpha, interleukin-1 beta) and adhesion molecules in our model. HO-1 could exert both anti-apoptotic and anti-inflammatory effects.20-22 One of the possible mechanisms by which HO-1 mediated both of these effects was associated with the activation of the mitogen-activated protein kinase p38 pathway. HO-1 and its catalytic product, carbon monoxide, were able to suppress the tumor necrosis factor-alpha-mediated endothelial cell apoptosis by activating phosphorylation of p38.20 Specific inhibition of p38 activity could abolish the anti-apoptotic effects of HO-1. Similar results were also observed in the studies on macrophages,22 in which HO-1 suppressed the production of pro-inflammatory cytokines in a p38-dependent manner. In the majority of studies, carbon monoxide was shown to be an important mediator of HO-1 effects.22, 23
Although it is possible that modulation of host immune response is partly through attenuation of intragraft inflammatory response by the expression of HO-1, accumulated evidences have shown that HO-1 can directly modulate immune response. Prolongation of allograft survival could be achieved by administration of the HO-1 inducer (cobalt protoporphyrin) or HO-1 gene transfer.14, 24-26 This effect could be substituted by giving the catalytic products of HO-1 to the recipients, including carbon monoxide and biliverdin/bilirubin.23, 27 Among the 3 catalytic products, carbon monoxide28 and biliverdin were able to suppress T cell activation and proliferation by suppressing the activities of the 2 transcription factors, NFkB and NFAT, in an in vitro model (Yamashita et al.,29). In addition, the anti-proliferative effects of rapamycin was found to be mediated by the expression of HO-1,30 suggesting that HO-1 could act as a regulator in the control of T cell proliferation through suppressing the mammalian target of rapamycin (mTOR) pathway. This hypothesis was supported by the evidence that HO-1 could inhibit the number of interferon-γ or interleukin-4 producing CD4+CD25+ cells in vivo (Tsui et al., unpublished observations).
The reason why HO-1 over-expression functioned more efficiently in small-for-size allografts remains to be determined. One possible reason was that the initiation of acute rejection process in small-for-size allografts might be more related to injury and inflammatory reaction than in the whole allografts (Yang et al.31), as the apoptotic and necrotic cells were found to be more immunogenic32 and regenerating liver grafts might present more alloantigens.6 In addition, early up-regulation of adhesion molecules in the small-for-size allografts could also trigger lymphocyte migration and activation. Therefore, early intervention of the injury and inflammatory processes might alter alloantigen recognition, activation of antigen presenting cells, and subsequent cell infiltration. Although we could also achieve survival prolongation in both whole and small-for-size allografts by AdHO-1 after reperfusion, the survival time was much shorter than pretreatment. This might indicate that conditional over-expression of HO-1 was crucial in protecting allografts, especially for small-for-size allografts, in which a more severe injury occurred. Another explanation might be related to the difference of the ability in the induction of HO-1 expression between whole and small-for-size allografts: the small-for-size allografts displayed a relatively higher level of HO-1 expression and its enzymatic activity after AdHO-1 administration than whole allografts.
Taken together, our data provided direct evidence to clarify the functional role of HO-1 in the small-for-size liver grafts, especially small-for-size allografts. The higher expression level of HO-1 accompanied with more severe liver dysfunction did not indicate the cytotoxicity of HO-1 in the small-for-size allografts. However, it did suggest the possibility that there was insufficiency of endogenous HO-1 in overcoming liver injury during the early post-transplant phase, since further induction of HO-1 expression significantly improved the liver function of the small-for-size allografts. In direct comparison of HO-1 over-expressing whole and small-for-size allografts, HO-1 could better exert its beneficial effects on the prolongation of small-for-size allograft survival. Although a precise mechanism is still not known, evidence showing better suppression of adhesion molecules in the small-for-size allografts suggests that the ability of HO-1 induction and the level of HO-1 expression can determine the liver allograft survival.