Tailoring donor hepatectomy per segment 4 venous drainage in right lobe live donor liver transplantation
Abstract
Including the middle hepatic vein (MHV) in the right lobe liver graft for adult-to-adult live donor liver transplantation provides more functional liver by securing adequate venous drainage. Donor outcome of this procedure in relation to different venous drainage patterns of segment 4 is unknown. Modification of graft harvesting technique by preserving segment 4b hepatic vein (V4b) in theory compensates for unfavorable venous drainage patterns. Consecutive 120 right lobe live donors were included. Computed tomography was studied in detail to assign each donor to one of the three types of the Nakamura classification of venous drainage pattern of segment 4. Type I drainage was mainly via the left hepatic vein (LHV), type II drainage was equally into the MHV and LHV, and type III drainage was predominantly into the MHV. Any distinct umbilical vein was also noted. In the early part of the series, the V4b draining into the MHV was divided to provide a long MHV stump in the graft. In the later part of the series, prominent V4b draining into the MHV was preserved in the donor as far as possible. Donor outcomes were measured by peak values of prothrombin time (PT), serum bilirubin and transaminases levels. There was no donor mortality. Type I donors (n=69) had the best outcome with peak PT of 17.9 sec (range 12.3–23.3 sec). Type II donors (n=44) had peak PT of 18.5 sec (range 15.4–24.4 sec). When V4b was preserved in type II donors (n=19), the peak PT (18.0 sec, range 15.4–20.7 sec) became significantly lower than that of type II donors who had V4b sacrificed (20.3 sec, range 16.2–24.4 sec) (P=0.001). A distinct umbilical vein (n=91, 75.8%) was insignificant for donor outcome measured by peak PT. Multivariate analysis identified that type II donors with V4b sacrificed (n=25), type III donors (n=7), and the first 50 cases had less favorable outcomes. In conclusion, unfavorable venous drainage patterns were one of the independent factors compromising postoperative donor liver function, but was circumvented by preservation of V4b. (Liver Transpl 2004;10:755–762.)
Live donor liver transplantation (LDLT) has become a powerful treatment modality for selected patients with end-stage liver diseases. In adult-to-adult LDLT, the right lobe is utilized in order to provide a graft of adequate volume. Including the middle hepatic vein (MHV) in the right lobe graft secures venous drainage of the right anterior sector. Such a radical graft design remains controversial as this renders the remnant left lobe devoid of drainage by the MHV. The estimated incidence of death among right lobe donors is about 1%.1 Although a recent survey in the United States demonstrated a lower donor death rate (0.2%), catastrophic outcome of donors developing liver failure and requiring liver transplantation has been reported.2 Apart from bleeding and cardiovascular complications, the most predictable mode of donor mortality is liver failure as a result of inadequate remnant liver volume. Deprivation of venous drainage of segment 4 via the MHV impedes the hypertrophy of segment 4 of the remnant left lobe.3 It is nonetheless not known how well the remnant left lobe functions in the absence of the MHV, especially in relation to the different venous drainage patterns of segment 4. Segment 4 venous drainage is via both the MHV and left hepatic vein (LHV). Nakamura identified that there could be three types of drainage pattern.4 Type I pattern is the most common (61.9%) and drainage is predominantly into the LHV. Type II pattern consists of venous drainage evenly into the LHV and the MHV (28.6%). Least common (9.5%) is type III pattern when the drainage is predominantly into the MHV (Fig. 1). This study evaluates the outcomes of a consecutive cohort of 120 donors of adult-to-adult right lobe LDLT, all including the MHV in respect to the venous drainage pattern of segment 4. Furthermore, the question of whether modification of graft harvesting technique according to the anatomical configuration of venous drainage of the segment 4 can improve safety margin of the donors is also examined.
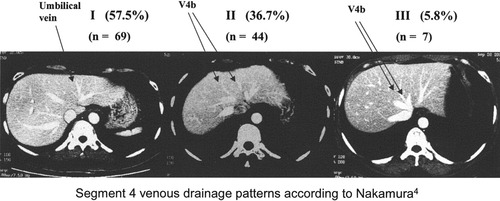
Computed tomography of donor liver with different patterns of segment 4 venous drainage and the case distribution in this series of 120 donors.
Abbreviations
MVH, middle hepatic vein; V4b, segment 4b hepatic vein; LHV, left hepatic vein; PT, prothrombin time; LDLT, live donor liver transplantation; CT, computed tomography; ESLV, estimated standard liver volume; RPV, right portal vein; RHA, right hepatic artery; HTK, histidine-tryptophan-ketoglutarate;ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio.
Materials and Methods
From May 1996 to February 2003, 120 donors of LDLT underwent donor right hepatectomy including the MHV at the University of Hong Kong Medical Centre, Queen Mary Hospital, Hong Kong. In stage 1 of the work-up, they were counseled by the surgeon, focusing on the indication and outcome of liver transplantation of the recipient. A 5-year survival rate of 85% and hospital mortality rate of 5% of the recipients were explained. Details of the donor operation, a complication rate of 15%, and an estimated mortality rate of 1% were elucidated. The voluntary nature of the donor's initiative was emphasized. Should these donors maintain the initiative, and true voluntarism reasonably ensured, they would proceed to stage 2 of the work-up.
The stage 2 work-up included blood tests for hematology, liver, renal biochemistry, and viral serology screening. If they were ABO compatible and other blood test results were satisfactory, they would proceed to a careful psychological assessment by the clinical psychologist to identify any contraindications. A three-phase contrast-enhanced computed tomogram (CT) of the liver was made. Tracings were made on the liver image with the transection line along the MHV. Volume of the right lobe including the MHV, the left lobe, and the caudate lobe, was measured by computer program based on the principle of Heymsfield.5 The donors were considered suitable for liver donation if the estimated right lobe graft volume was more than 40% of the estimated standard liver volume (ESLV) of the recipient, as calculated by the formula of Urata et al.6 The minimal volume required for the remnant left lobe was 30%. Selective angiograms of the celiac trunk and superior mesenteric artery were then performed, mainly to identify the origin of the segment 4 hepatic artery, and to demonstrate any vascular anomalies requiring modification of technique. This artery is identified and protected during the operation in all cases to maintain arterial supply of segment 4. Otherwise, segment 4 infarction would impose more risks on the donor.
The CT scan was studied meticulously with special reference to the anatomical arrangement of the hepatic veins and their tributaries. Special attention was paid to identify the segment 4b hepatic veins (V4b), which drain the cranial portion of the segment 4 into either the MHV or LHV, and the umbilical vein.7, 8 The umbilical vein, also called the scissural vein,7 and the left medial vein,9 is a tributary of the LHV. It is recognized on the CT by its course beneath the falciform ligament and in line with the umbilical fissure. Serial axial cuts of the CT were examined in order to determine which of the three categories of the Nakamura classification of segment 4 venous drainage pattern the donor conformed to (Fig. 1). The classification was made by identification of the prominent V4b and tracing the course of these prominent veins into the MHV, LHV, or both. In the event of two or more distinct V4b draining into either the MHV or LHV, the size of the predominant vein determined the classification.
Donor operation has been described in detail elsewhere.10, 11 The abdomen was explored through a bilateral subcostal incision with midline cephalic extension. Cholecystectomy was performed followed by operative cholangiography to delineate the biliary anatomy. The right hepatic artery and right portal veins were isolated. The right lobe of the liver was mobilized and freed from the inferior vena cava. The right hepatic vein was isolated. Parenchymal transection was performed along the line of demarcation produced by temporary clamping of the right portal vein (RPV) and right hepatic artery (RHA). Inflow control by Pringle maneuver was not employed in any of these donors. The MHV was included in the right-lobe liver graft and the transection plane was exactly along the Cantlie's line using the Cavitron Ultrasonic Surgical Aspirator (CUSA System 200; Valleylab Inc., Boulder, CO), exposing the left side of the MHV. In the early part of the series, the MHV was skeletonized at the region of the root with the inferior vena cava or LHV by dividing and ligation of all V4b because we thought that successful MHV reconstruction required a long MHV stump in the graft. In the later part of the series, a major V4b draining into the MHV near its root was preserved and the transection of the MHV was made caudal to this V4b (Fig. 2). The RPV was cannulated and divided. The RHA was clamped and divided. The MHV and RHV were stapled with a vascular stapler (Premium Multifire TA30, Tyco Healthcare Group, Norwalk, CT, or ATW 35, Ethicon Endo-surgery, Inc, Cincinnati, OH) and then divided. On the back-table the graft was flushed with either University of Wisconsin solution (UW; Viaspan, Du Pont Pharmaceuticals, Wilmington, DE), or, from case 100, with histidine-tryptophan-ketoglutarate (HTK; Custodiol, Köhler Chemie GmbH, Alsbach, Germany). Venoplasty of the MHV and RHV was performed on the back-table from case 86 onward.12
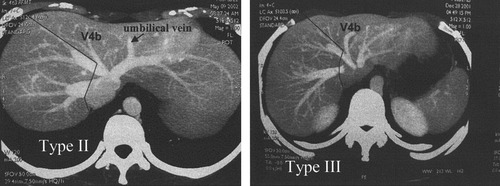
Proposed line of liver transection to preserve V4b in Nakamura type II and type III livers.
Following retrieval of the right lobe liver graft, intraoperative Doppler ultrasonography was repeated to ascertain integrity of the left hepatic artery, left portal vein, and left hepatic vein, and to study the flow pattern in the segment 4 portal vein. Methylene blue was instilled into the biliary system gently to identify any site of bile leakage. The falciform ligament was sutured to the anterior abdominal wall to prevent rotation and outflow obstruction of the remnant left lobe.13 No abdominal drain was used from the 25th donor. Donor outcomes including intensive care unit stay, hospital stay, and complications were documented in detail prospectively. The peak prothrombin time (PT), serum bilirubin, serum alanine aminotransferase (ALT), and aspartate aminotransferase (AST) over the entire hospitalization period were recorded. The degree of fatty change of the liver graft as estimated by histological examination of liver biopsy was obtained after graft reperfusion and graded by D'Alessandro's Classification: nil (0%), grade 1a (≤10%), grade 1b (11–30%), grade 2 (31–60%), grade 3 (>60%).14
Postoperatively, the donors were nursed in the intensive care unit. Routine computed tomography or ultrasonography were not performed on the donors. In this study, all values were expressed as median and range. The chi-squared test was used for comparisons of discrete variables and the Mann-Whitney U test was used for comparison of continuous variables. P<0.05 was considered statistically significant, and all tests were two-tailed. Variables with P<0.1 in the univariate analysis were then included in a forward, stepwise multiple logistic-regression model to identify the most important risk factors for adverse donor outcomes. Statistical analysis was performed using SPSS 10.0 for Windows computer software (Statistical Package for the Social Sciences, Chicago, IL).
Results
Demographic and Clinical Data
The median age of these 120 donors was 37 years (range, 18–57 years). Spouses accounted for 40% of the donors, followed by offspring (25.8%), siblings (15.0%), other relatives (9.9%), parents (5.9%), and friends (3.3%). All except two were Chinese. The median body mass index was low at 21.5 (range, 16.5–35.6). Sixty-eight (56.7%) donors had no fatty change, 45 (37.5%) patients had grade 1a and 7 (5.8%) had grade 1b fatty change. The median left lobe to total liver volume ratio was 34.0% (range, 23.6–45.2%). Graft volume to ESLV ratio was 48.7% (range 32.3–89%). Drainage pattern of segment 4 was most commonly type I (69 donors, 57.5%), followed by type II (44 donors, 36.7%), and type III (7 donors, 5.8%) (Fig. 1).
Ninety-one (75.8%) donors had a distinct umbilical vein. In the 7 type III donors, only 4 (57.1%) had a distinct umbilical vein. Fifty-eight donors were identified to have the V4b sacrificed (Table 1). Among these 58 donors, 29 were in the first 50 cases (58.0%) and 29 were in the subsequent 70 case (41.4%). The proportion of donors with the umbilical vein, and V4b sacrificed in different categories of Nakamura type venous drainage pattern is listed in Table 1.
| Distinct Umbilical Vein Present | V4b Draining into MHV Sacrificed | Peak PT (Sec) | Peak Bilirubin (μmol/L) | Peak ALT (U/L) | Peak AST (U/L) | |
|---|---|---|---|---|---|---|
| Type I n = 69 | 52 (75.4%) | 29 (55.8%) | 17.6 (12.3–23.2)* | 49 (23–234) | 180 (76–838) | 199 (91–832) |
| Type II n = 44 | 35 (79.5%) | 25 (56.8%) | 18.5 (15.4–24.4) | 59 (10–203) | 199 (73–816) | 217 (110–682) |
| Type III n = 7 | 4 (57.1%) | 4 (57.1%) | 17.8 (14.5–21.9) | 61 (44–70) | 159 (111–439) | 208 (124–447) |
- Abbreviations: MHV, middle hepatic vein; PT, prothrombin time; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
- * P = 0.006 vs. Type II.
- Continuous data are expressed as median and range.
Outcome
The median blood loss volume was 410 mL (range 42–1600 mL). Only one donor required blood transfusion (1 unit) and 2 donors, both type I, received fresh frozen plasma on the first postoperative day (1,040 mL and 540 mL), for a PT level of 17.3–19.5 sec and 23.2 sec respectively. Period of stay in the intensive care unit was 2 days (range, 0–8 days). Over the entire hospitalization period of the donors (median, 9 days; range, 5–38 days), the median peak international normalized ratio (INR) was 1.5 (range, 1.2–2.0), median peak PT was 18.0 sec (range, 12.3–24.4 sec), median peak serum bilirubin level was 52 umol/L (range, 10–234 umol/L), median peak ALT level was 183 U/L (range, 73–838 U/L), and the median peak AST level was 204 U/L (range, 91–832 U/L). There was no donor mortality. Twenty-eight (23.3%) donors had one or more complications. The most common complication was wound infection (12.5%).
Type I donors (n = 69) had the best outcome with peak PT of 17.6 sec (range, 12.3–23.2 sec). Type II donors (n = 44) had peak PT of 18.5 sec (range, 15.4–24.4 sec) (Table 1). The result of the peak INR values was similar to that of the peak PT. The PT from postoperative day 1 to day 3 was significantly longer in type II donors (Fig. 3). The serum levels of ALT and AST were not significantly different except for AST on postoperative days 2 and 3. No significant difference was found in the peak values of PT, bilirubin, ALT, or AST in donors with or without a distinct umbilical vein (Table 2). When in type II donors V4b draining into the MHV was sacrificed, the peak PT (20.3 sec) was significantly higher than that in type II donors with preserved V4b (18 sec) (P = 0.001) (Table 3). In the Type II donors with preserved V4b the peak PT was similar to that in the type I donors (17.9 sec).
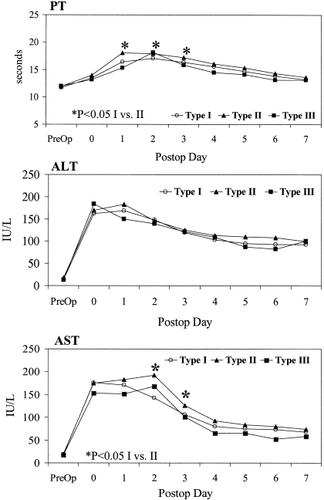
Graphs showing the median prothrombin time, median serum alanine aminotransferase levels, and median serum aspartate aminotransferase levels of donors.
| Peak PT (sec) | Peak Bilirubin (μmol/L) | Peak ALT (U/L) | Peak AST (U/L) | |
|---|---|---|---|---|
| Distinct umbilical vein present n = 91 (75.8%) | 18.0 (12.3–24.4) | 52 (13–234) | 180 (73–838) | 200 (109–832) |
| No distinct umbilical vein n = 29 (24.2%) | 18.4 (14.5–23.2) | 52 (10–203) | 192 (79–816) | 210 (91–832) |
- Abbreviations: PT, prothrombin time; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
- Continuous data are expressed as median and range.
| Peak PT (sec) | Peak Bilirubin (μmol/L) | Peak ALT (U/L) | Peak AST (U/L) | |
|---|---|---|---|---|
| Nakamura I V4b preserved (n = 40) | 17.9 (12.3–22.3) | 52 (23–234) | 181 (76–647) | 205 (91–514) |
| Nakamura I V4b sacrificed (n = 29) | 17.5 (15.0–23.2) | 44 (26–124) | 180 (101–838) | 181 (109–832) |
| P value | 0.688 | 0.412 | 0.378 | 0.053 |
| Nakamura II V4b preserved (n = 19) | 18.0 (15.4–20.7) | 56 (10–203) | 190 (101–816) | 221 (116–682) |
| Nakamura II V4b sacrificed (n = 25) | 20.3 (16.2–24.4) | 61 (24–131) | 208 (73–725) | 202 (110–515) |
| P value | 0.001* | 0.943 | 0.804 | 0.636 |
| Nakamura III V4b preserved (n = 3) | 17.8 (17.5–21.9) | 52 (48–64) | 215 (111–439) | 271 (224–447) |
| Nakamura III V4b sacrificed (n = 4) | 18.2 (14.5–21.2) | 64 (44–70) | 148 (116–226) | 185 (124–208) |
| P value | 0.480 | 0.480 | 0.724 | 0.034* |
- Abbreviations: PT, prothrombin time; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
- Continuous data are expressed as median and range.
- * P < 0.05.
The peak PT of the combined groups of type II donors with V4b sacrificed (n = 32) and Type III donors (n = 7) was found to be significantly higher than that in the combined goups of type I donors (n = 69) and type II donors with V4b preserved (n = 19) (P = 0.001) (Table 4). The PT values during the first 7 postoperative days were found to be significanlty different between these two groups (Figure 4). However, the ALT and AST values were not different. In the last 74 consecutive donors, intraoperative Doppler ultrasonography of the segment 4 portal vein, performed after the removal of the right lobe graft, showed normal flow direction in 44 (59.5%) donors, static flow in 13 (18%) donors and reversed flow in 17 (23%) donors. However, no correlation was found between the flow pattern in segment 4 portal vein and the hepatic venous drainage pattern of segment 4.
| Peak PT (Sec) | Peak Bilirubin (μmol/L) | Peak ALT (U/L) | Peak AST (U/L) | |
|---|---|---|---|---|
| Nakamura I & Nakamura II V4b preserved n = 88 | 17.9 (12.3–23.2) | 50 (10–234) | 182 (76–838) | 204 (91–832) |
| Nakamura II V4b sacrificed & III n = 32 | 20.3 (14.5–24.4) | 61 (24–131) | 196 (73–725) | 205 (110–515) |
| P value | 0.000* | 0.252 | 0.799 | 0.601 |
- Abbreviations: PT, prothrombin time; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
- Continuous data are expressed as median and range.
- * P < 0.005
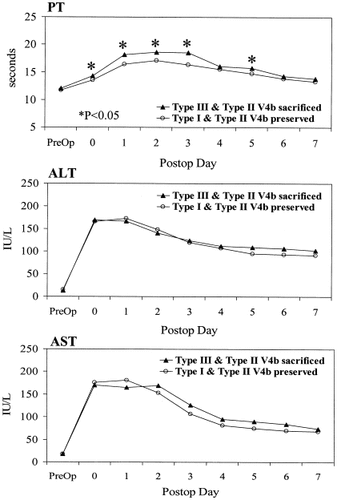
Graphs showing the median prothrombin time, median serum alanine aminotransferase levels, and median serum aspartate aminotransferase levels of donors.
Learning Curve
In order to evaluate the effect of the learning curve, the operation time was plotted against the case number (Figure 5). After the first 50 cases the operating time appeared to become stable between 400–500 minutes. The postoperative peak PT of the first 50 cases was compared with the latter 70 cases.
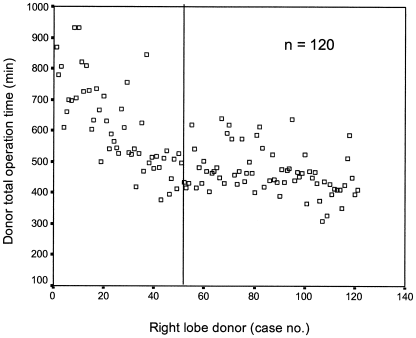
Graph plotting operation time against case number of donor.
Adverse Factors
Adverse outcome for the donor was defined as a peak PT longer than 18.0 sec. In general, for patients with major hepatic resections, fresh frozen plasma infusion has been recommended for a PT between 16–18 secs.15 We upheld the policy not to give blood products to the donor until deemed clinically necessary. Possible variables for adverse donor outcome were subjected to univariate analysis. (Table 5). These factors were age, gender, operation time, blood loss, segment 4 drainage pattern, first 50 cases, left lobe to whole liver less than 34%, fatty change over 10%, and body mass index over 21.5%. Factors with a P-value <0.1 were then subjected to multivariate analysis. These factors were: age, operation time, segment 4 drainage pattern, first 50 cases, and body mass index. By multivariate analysis, drainage pattern to type II with V4b sacrificed and type III (relative risk 2.736, 95% CI, 1.120–6.688, P = 0.027), and the first 50 cases (relative risk 2.555, 95% CI, 1.158–5.638, P = 0.020) were the independent predictive factors for adverse outcome.
| PT ≤ 18 sec (n = 62) | PT > 18 sec (n = 58) | P Value | |
|---|---|---|---|
| Age (yr) | 41 (18–57) | 35 (18–56) | .09 |
| Gender ratio (male:female) | 25:37 | 25:33 | .76 |
| Operation time (min) | 470 (310–930) | 509 (388–932) | .03* |
| Blood loss (ml) | 376 (80–1600) | 455 (42–1400) | .47 |
| Type I and Type II preserved V4b: Type II sacrificed V4b & Type III | 51:11 | 37:21 | .02* |
| First 50 cases: later 70 cases | 18:44 | 32:26 | .004* |
| Remnant left lobe (% of whole liver) | 34.0 | 33.9 | .46 |
| Fatty change >10%: ≤10% | 2:60 | 5:53 | .26 |
| Body mass index (kg/m2) | 22.3 (17.6–27.0) | 20.6 (16.5–35.6) | .05 |
- Abbreviation: PT, prothrombin time.
- Continuous data are expressed as median and range, otherwise figures represent number of patients.
- * P < 0.05.
Discussion
Unfavorable venous drainage pattern of the remnant left lobe of type II donors, when compounded by the sacrifice of V4b draining into the MHV, resulted in the longest peak PT of 20.3 sec (range, 16.2–24.4 sec). To be certain that the remnant left lobe can meet the metabolic demand of the donor, venous drainage of the left lobe must be sufficient and strategy to preserve the venous drainage must be exercised.
In this study, we focused on the consequences on the donor deprived of segment 4 venous drainage via the MHV. The venous drainage patterns of segment 4, whether evenly to the LHV and MHV, or preferentially to one of them were examined specifically. The peak levels of PT, bilirubin, ALT, and AST were used as surrogate markers of liver functions of the remnant left lobe. In terms of peak PT, the best performers were those with Nakamura type I segment 4 venous drainage where it was preferentially into the LHV. Second was in type II where it was preferentially into the MHV. The small number (n = 7) of donors in type III might not reflect the real situation in this category. Intermediate results were those of type II venous drainage pattern. Nonetheless, when V4b was sacrificed in type II donors, the liver function of the remnant left lobe was compromised. On the other hand, preserving V4b circumvented this problem.
Intuitively, a distinct umbilical vein was considered beneficial to venous drainage of segment 4. In this series, its presence did not seem to improve the outcome in terms of synthetic functions (Table 3). The existence of the umbilical vein per se was of less importance than the preferential drainage to LHV, i.e., Nakamura type I.
In this study, remnant left lobe volume was not an independent factor adversely affecting the outcome. Although we advocated that the donor should have at least a remnant liver volume of 30% before acceptance for donation, donors with remnant liver volume <30%16 underwent donor hepatectomy because of the critical condition of the recipient. In previous reports, a volume of 25%17 to 27%18 normal liver was considered adequate for a major hepatectomy in the normal liver. We, however, opted for a larger remnant liver volume to ensure safety for the donor. Nevertheless, in cases of smaller remnant liver volume, with attention to reduce operation time and blood loss, the donors could survive the operation.
Nakamura Type III is in theory the most unfavorable pattern, as drainage is preferentially into the MHV. Characteristically, the V4b in this category is distinct on intraoperative ultrasonography and readily identified on approaching the root of the MHV during parenchymal transection. Adhering to the policy of transecting the MHV caudal to a large V4b, the donors of type II or III should not be contraindicated for donor hepatectomy including the MHV. However, should this surgical plan not executed precisely, the donor is subjected to a higher risk. A prominent but too caudally located V4b, however, may not allow its preservation because the transection line of the MHV has to become very caudal. Nevertheless, in this situation, it is technically feasible to lengthen the MHV at the back-table using a piece of vein graft.19 Using the venoplasty technique,12 it is possible to ensure a patent MHV and uniform venous drainage of the right anterior sector of the graft. In the donor, as a congested portion of the remnant liver may enhance systemic inflammatory responses, careful preoperative appraisal of the CT in the portal venous phase and intraoperative navigation by ultrasonography to determine the exact dividing line of the MHV are essential.
In conclusion, in donor right hepatectomy including the MHV, unfavorable venous drainage pattern is an independent factor compromising postoperative liver function. Preservation of V4b vein in unfavorable cases circumvents this problem. This is part of the endeavor in keeping a wide margin of safety for the donor who voluntarily becomes a patient in the act of being a live liver donor.