Impact of long-term nasal airflow deprivation on sinonasal structures and chronic rhinosinusitis in total laryngectomy patients
Abstract
Objective
Total laryngectomy (TL) patients are good models in which to evaluate the effects of nasal airflow cessation on the sinonasal tract. Here, we evaluated changes in sinonasal structures and association with sinus opacification in the computed tomography (CT) images 3 years post-TL.
Methods
Patients who underwent TL from 2005 to 2017 in a teaching academic center were reviewed retrospectively. Patients with a final follow-up CT taken less than 3 years after TL, tracheoesophageal puncture, inadequate CT image, or history of sinonasal surgery were excluded. The control group included patients who underwent a partial laryngectomy or hypopharyngectomy without requiring a tracheotomy for more than a month. Altogether, 45 TL patients and 38 controls were selected. The volume of all four paranasal sinuses, inferior turbinate soft tissue volume (ITSTV), maxillary sinus natural ostium (MSNO) mucosal width, and Lund–Mackay scores (LMS) were measured on preoperative and postoperative CT scans.
Results
The mean duration between surgery and the final CT scan was 6.3 ± 2.4 and 5.5 ± 2.3 years for the TL and control groups, respectively. Neither group showed significant changes in the four paranasal sinuses' volume or MSNO mucosa width. The ITSTV decreased significantly, from 4.6 ± 1.3 to 2.8 ± 1.1 mL (p < .001), in the TL group, regardless of the presence of nasal septal deviation, showing ITSTV reduction on both concave and convex sides. By contrast, the control group showed no significant changes in ITSTV. Postoperative LMS changes in both groups were insignificant. The number of patients with LMS aggravation or alleviation was the same in both groups, regardless of preoperative sinus opacification.
Conclusions
Paranasal sinus structures and sinus opacification are not affected significantly by nasal airflow cessation; however, the inferior turbinate mucosa is affected by long-term discontinuation of nasal airflow.
Level of Evidence
4 (case–control study).
1 INTRODUCTION
The impact of nasal airflow on the nasal mucosa, as well as its association with chronic rhinosinusitis (CRS), has been the focus of interest for many otorhinolaryngologists. Previously, using an experimental rat model, an increase in goblet cells and ciliated nasal epithelial cells on the side of the surgically closed nostril was reported.1-3 Meanwhile, a restoration of the nasal mucosa was observed, followed by nostril obliteration in patients with atrophic rhinitis.4, 5 These results suggest a causative effect of increased nasal airflow on the nasal mucosa. However, previous publications did not use a normal, healthy human nasal mucosa as a study model, thereby limiting the applicability for determining the impact of nasal airflow on the human nasal mucosa.
In patients with very extensive or recurrent malignant tumors of the larynx or hypopharyngeal area, total laryngectomy (TL) is recommended.6 The nasal airway passage of patients who undergo TL is permanently disconnected from the tracheopulmonary airway.6 Therefore, the inhalation and expiration of the gas is achieved through the tracheotomy stoma, resulting in the complete cessation of the nasal airflow.7 Therefore, TL patients serve as an adequate model for evaluating the effects of nasal airflow deprivation in the pathophysiology of various sinonasal disorders and changes in the human nasal mucosa.
The effect of nasal airflow cessation using TL patient models were studied in many previously published literature. However, previous publications have only focused on the changes in the nasal mucosa histology, cytology, or ciliary function.8-10 Nevertheless, the previous publications did not meet a consensus in terms of whether atrophy or hypertrophic changes in the nasal mucosa following TL. In addition, there have been only two publications regarding the effect of nasal airflow discontinuation in the development of CRS, in which the ununited results were shown in two publications.11, 12
In the previous literature, no studies were conducted with TL patients with a minimal time interval of 3 years following TL to see the effect of nasal airflow cessation, lacking a long-term outcome. Also, up to the authors' knowledge, no previous studies with TL patients reported changes in the paranasal sinus volumes following TL. Therefore, the authors aimed to analyze the changes in the inferior turbinate soft tissue volume (ITSTV), paranasal sinus volume, as well as its association with CRS, with a minimum follow-up period of 3 years following TL.
2 MATERIALS AND METHODS
This study was approved by the institutional review board of Asan Medical Center (investigation no. 2021-0958).
2.1 Study subjects
A flowchart on the study subject recruitment is illustrated in Figure 1. Patients who have received TL in the Department of Otorhinolaryngology at Asan Medical Center from January 2005 to December 2017 have been retrospectively reviewed (Figure 1, left). All TL cases were performed due to the malignant neoplasm originating from the larynx and hypopharynx. All patients received TL with simultaneous unilateral or bilateral cervical lymph node dissection, and in cases with hypopharynx cancer or extensive lesion, simultaneous resection of the hypopharynx and cervical esophagus was performed. Patients with a final follow-up computed tomography (CT) image less than 3 years after TL were excluded from the study. Patients with a history of previous sinonasal surgery and a CT image only confined to the cervical region, excluding the paranasal sinus, were excluded as well. Altogether, 45 patients were finally enrolled in the “TL group.”
To determine the impact of nasal airflow discontinuation on the sinonasal structures, a control group of similar surgical extent with a similar disease burden to the TL group was recruited. The authors defined the control group as patients who have undergone partial laryngectomy or partial hypopharyngectomy with simultaneous cervical lymph node dissection, who did not require initial tracheotomy, or patients able to decannulate during the first 1-month postoperation. From 2005 to 2015, 71 patients underwent partial resection of the larynx or hypopharynx with simultaneous cervical node dissection (Figure 1, right). Similar to the TL group, patients with final CT images less than 3 years following surgery or CT images only confined to the cervical area were excluded from the study. Altogether, 38 patients were selected for the control group. The clinical characteristics and demographics of both TL and control groups were reviewed, as well as the information regarding the nutritional status represented as body mass index and serum protein and albumin levels,12 tobacco smoking history, and whether the adjuvant chemo- or radiation therapy were given were collected.
2.2 CT image analysis
A contrast-enhanced CT scan of the head and neck area was performed in all patients using the same protocol. From the vertex to the upper sternum, consecutive scans of 0.6 mm were taken following an image reconstruction. All CT analysis was performed on a window setting of window width of 300 and window level of 30.
To determine changes in the sinonasal structures, the authors planned to evaluate the changes in the paranasal sinus volume, soft tissue volume of the IT, and the width of the maxillary sinus natural ostium (MSNO) mucosa. All CT image measurements were delivered by two board-certified otorhinolaryngologists (M.J.P. and M.B.), with blinding of the clinical information. For the preoperative and postoperative comparison, a CT image taken in the preoperative period was compared with the most recent (final) CT image in each patient.
To measure the volume of the four paranasal sinus skeletons (maxillary, ethmoid, frontal, and sphenoid), the inner bony cortical lining of each paranasal sinus was traced, and the surface of the traced region was measured on the axial CT cut (Figure 2A). Afterward, the volume of each paranasal sinus was calculated with the planimetry method using the PetaVision for Clinics (ver.3.1.0) software (PetaVision, Asan Medical Center, Seoul, Korea) in parallel with the previously published methods.13
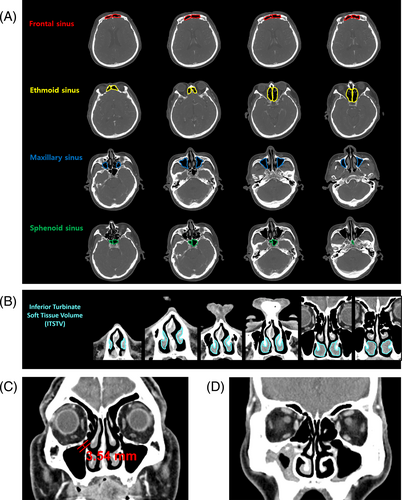
The ITSTV was measured in a manner similar to that for measuring paranasal sinus volume. On the coronal CT image, the outer lining of the IT was traced, while the bony portion of the IT was excluded, followed by volume calculation of the IT soft tissue (Figure 2B). For the MSNO mucosal width measurement, MSNO was identified on the coronal CT image, and the width of the MSNO mucosa was measured (Figure 2C). For complete ostiomeatal obstruction, the MSNO mucosal width was considered 0 mm. To evaluate the severity of CRS, a Lund–Mackay score (LMS)14 was adopted, as in previous literature,11, 15 with a score range from 0 to 24, representing the degree of CRS (Figure 2D).
In addition, to evaluate the difference in sinonasal microstructure following nasal airflow cessation according to the presence of nasal septal deviation (NSD), NSD was assessed as follows: the most deviated point of the nasal septum on the coronal CT image where the crista gali was present was marked. The angle formed by the lines from the marked point to the center of the cribriform plate and maxillary crest was measured. A patient was considered to have an NSD when the measured angle showed more than 170°, as cited in previous publications.16, 17
2.3 Statistical analysis
To analyze the differences in the clinical characteristics between the TL and control groups, the t-test, chi-squared test, and Fisher's exact test were adopted. In cases where there are less than 5 or no observations in any individual cell, the chi-squared test was replaced with a Fisher's exact test for testing independence to provide a greater accuracy. To evaluate the statistical significance between the preoperative and postoperative values, a paired t-test was adopted. All statistical analyses were performed in a two-tailed manner. A p < .05 was considered statistically significant. All statistical analyses were performed using SPSS ver. 22 (IBM Corp., Armonk, NY, USA).
3 RESULTS
No significant differences in clinical features were noted between the TL and control groups (Table 1). The TL group had 1 (2.2%) female patient, whereas the control group had all male patients. The mean duration of the time interval between surgery and final CT image follow-up was 6.3 ± 2.4 years in the TL group and 5.5 ± 2.3 years in the control group, showing no statistical difference. The number of preoperative CRS (preoperative LMS > 0) was 12 (26.7%) in the TL group and 11 (28.9%) patients had positive preoperative LMS in the control group.
| Total laryngectomy group (N = 45) | Control group (N = 38) | p Valuea | |
|---|---|---|---|
| Mean ± SD (min–max) | |||
| Age (at the time of total or partial laryngectomy) | 61.6 ± 9.0 (44–77) | 63.2 ± 9.4 (34–84) | .520 |
| Time interval between the preoperative CT image and laryngectomy (days) | 27.7 ± 14.9 (1–63) | 22.8 ± 13.9 (2–72) | .144 |
| Time interval between the laryngectomy and final postoperative CT image (years) | 6.3 ± 2.4 (3–11) | 5.5 ± 2.3 (3–10) | .404 |
| Nutritional status (at the time of total or partial laryngectomy) | |||
| Initial body mass index (kg/m2) | 22.6 ± 2.2 | 22.4 ± 2.3 | .640 |
| Initial serum total protein (g/dL) | 6.5 ± 0.5 | 6.6 ± 0.6 | .379 |
| Initial serum albumin (g/dL) | 4.1 ± 0.6 | 4.1 ± 0.6 | .400 |
| N (%) | p Valueb | ||
| Sex (male/female) | 44/1 (97.8/2.2) | 38/0 (100/0) | 1.000 |
| History of tobacco smoking >10 pack years | 40 (88.9) | 36 (94.7) | .449 |
| Primary site of the malignant tumor | |||
| Glottis/hypopharynx | 38/7 (84.4/15.6) | 31/7 (81.6/18.4) | .121 |
| Presence of nasal septal deviation | 35 (70) | 28 (73.7) | .664 |
| Preoperative Lund–Mackay score >0 | 12 (26.7) | 11 (28.9) | .643 |
| Preoperative treatment modality | |||
| Radiation therapy | 10 (22.2) | 7 (18.4) | .483 |
| Chemoradiation therapy | 5 (11.1) | 1 (2.6) | .212 |
| Induction chemotherapy | 1 (2.2) | 0 (0) | 1.000 |
| Postoperative treatment modality | |||
| Adjuvant radiation therapy | 13 (28.9) | 7 (18.4) | .267 |
| Adjuvant chemoradiation therapy | 5 (11.1) | 6 (15.8) | .531 |
- a p Value calculated using the t-test between the total laryngectomy group and control group.
- b p Value calculated using the chi-square test or Fisher's exact test, between the total laryngectomy group and control group.
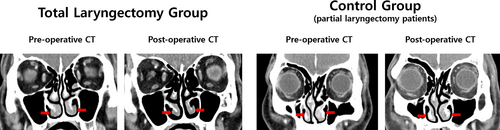
The volume of all four paranasal sinuses and MSNO mucosal width did not significantly change on postoperative CT image over 3 years following TL (Table 2) and the control group. However, the ITSTV measured on the CT image over 3 years following TL showed a significant decrease from 4.6 ± 1.3 to 2.8 ± 1.1 mL (p < .001), whereas the control group showed no significant changes in ITSTV.
| Total laryngectomy group (N = 45) | Control group (N = 38) | |||||
|---|---|---|---|---|---|---|
| Prior to total laryngectomy | >3 years after total laryngectomy | p Valuea | Prior to partial laryngectomy | >3 years after partial laryngectomy | p Valueb | |
| Paranasal sinus volume (mL)a | ||||||
| Maxillary sinus | 16.1 ± 5.2 | 16.1 ± 5.2 | .692 | 15.7 ± 4.7 | 15.7 ± 4.3 | .835 |
| Ethmoid sinus | 6.9 ± 2.2 | 7.2 ± 2.2 | .248 | 8.1 ± 2.7 | 8.0 ± 2.7 | .628 |
| Frontal sinus | 2.7 ± 1.9 | 2.8 ± 1.8 | .237 | 3.6 ± 2.0 | 3.6 ± 2.0 | .601 |
| Sphenoid sinus | 3.9 ± 1.6 | 3.9 ± 1.9 | .863 | 4.2 ± 1.7 | 4.2 ± 1.7 | .282 |
| Maxillary sinus natural ostium mucosal width (mm)a | 2.6 ± 1.2 | 2.9 ± 1.4 | .166 | 2.2 ± 0.9 | 2.2 ± 0.9 | .409 |
| Inferior turbinate soft tissue volume (mL)a | 4.6 ± 1.3 | 2.8 ± 1.1 | <.001 | 4.2 ± 1.7 | 4.1 ± 1.6 | .630 |
- Note: The mean of bilaterally measured value of each anthropometric value is shown. All values are represented as the mean ± SD.
- a p Value calculated with paired t-test between the preoperative measurements and final CT image over 3 years following total laryngectomy.
- b p Value calculated with paired t-test between the preoperative measurements and final CT image over 3 years following partial laryngectomy or partial hypopharyngectomy.
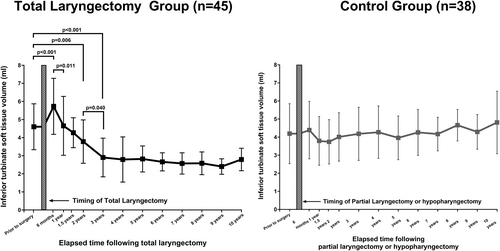
Among all CT-derived measurements, ITSTV only showed significant changes following TL. Therefore, an additional investigation on the ITSTV changes according to the time interval following TL was conducted (Figure 3). The mean preoperative ITSTV in the TL group was 4.6 ± 1.3 mL, which significantly increased to 5.7 ± 1.6 mL (p < .001) at the 6-month postoperative period. In the 1-year postoperative period, ITSTV significantly decreased to 4.6 ± 1.6 mL from 6 months following TL (p = .011), showing no significant difference from preoperative ITSTV. The 2- and 3-year postoperative ITSTV showed a significantly decreased ITSTV of 3.8 ± 1.2 mL (p = .006) and 2.9 ± 1.1 mL (p < .001), respectively, compared with preoperative ITSTV. The 2- and 3-year postoperative ITSTV showed significant differences (p = .040), whereas there were no significant differences between groups in the postoperative period over 3 years.
The mean preoperative LMS of TL and control groups were 0.6 ± 1.2 and 0.9 ± 1.9, respectively, with no significant difference (Table 3). In both TL and control groups, no significance in the LMS changes was noticed following surgery. Also, it was observed that the LMS remained consistent over time (Figure S1), and the fluctuations in LMS remained below an incidental score of LMS <5, as a LMS below 5 can be incidentally noticed in the previous literature,18 in almost all subjects following TL. Furthermore, there was no correlation between the severity or improvement of CRS and the preoperative LMS in both the TL and control groups (Figures S1 and S2). In the postoperative period, no patients have undergone functional sinus surgery for the management of sinusitis in all TL and control group patients.
| Lund–Mackay score (mean ± SD) | |||
|---|---|---|---|
| Prior to surgery | >3 years after surgery | p Valuea | |
| All patients | |||
| Total laryngectomy group (N = 45) | 0.6 ± 1.2 (0–5) | 0.7 ± 1.6 (0–7) | .947 |
| Control group (N = 33) | 0.9 ± 1.9 (0–9) | 0.8 ± 1.7 (0–9) | .181 |
| p Valueb | .533 | .842 | |
| Preoperative LMS >0 | |||
| Total laryngectomy group (N = 12) | 2.5 ± 1.1 (1–5) | 2.0 ± 1.9 (0–6) | .341 |
| Control group (N = 11) | 3.3 ± 2.2 (1–9) | 2.5 ± 2.4 (0–9) | .125 |
| p Valueb | .536 | .918 | |
| Preoperative LMS = 0 | |||
| Total laryngectomy group (N = 33) | 0 | 0.3 ± 1.2 (0–7) | .169 |
| Control group (N = 27) | 0 | 0.1 ± 0.3 (0–1) | .161 |
| p Valueb | – | .410 | |
- Note: Values represented in mean ± SD (min–max).
- a p Value calculated with paired t-test of LMS between the preoperative measurements and the final CT image over 3 years following surgery.
- b p Value calculated with chi-square test or Fisher's exact test between the total laryngectomy group and control group.
To evaluate the cessation of altered nasal airflow with NSD that might affect changes in ITSTV and MSNO mucosal width, a further breakdown of TL patients was made due to the presence of NSD (Table 4). Therefore, a significant reduction in ITSTV was seen in both patients with NSD (p < .001) and without NSD (p = .025), while MSNO mucosal width showed no significant changes, regardless of the presence of NSD. ITSTV was significantly reduced on both concave and convex nasal cavities (p < .001, both).
| N = 45 (total laryngectomy group) | Patients with NSD (N = 35) | Patients without NSD (N = 10) | ||||
|---|---|---|---|---|---|---|
| Prior to total laryngectomy | >3 years after total laryngectomy | p Valuea | Prior to total laryngectomy | >3 years after total laryngectomy | p Valuea | |
| Mean ITSTV (mL) | 4.78 ± 1.15 | 3.82 ± 1.11 | <.001 | 4.16 ± 1.48 | 3.61 ± 1.07 | .025 |
| Concave side IT soft tissue volume (mL) | 4.57 ± 1.36 | 3.72 ± 1.18 | <.001 | |||
| Convex side IT soft tissue volume (mL) | 5.01 ± 1.12 | 3.92 ± 1.14 | <.001 | |||
| Mean MSNO mucosal width (mm) | 2.44 ± 1.02 | 2.84 ± 1.30 | .068 | 3.29 ± 1.07 | 3.12 ± 1.43 | .723 |
| Concave side MSNO mucosal width (mm) | 2.43 ± 1.19 | 2.79 ± 1.45 | .101 | |||
| Convex side MSNO mucosal width (mm) | 2.46 ± 1.27 | 2.90 ± 1.39 | .117 | |||
- Note: All values are presented as the mean ± SD.
- a p Value calculated using a paired t-test (preoperative measurements compared with the final CT image over a period of 3 years following total or partial laryngectomy).
4 DISCUSSION
In the present study, we report the changes in the paranasal sinuses and sinonasal structures following TL in the postoperative CT image with a minimum of 3 years and a mean of 6 years of surveillance period in 45 TL patients. The volume of all four paranasal sinuses, MSNO mucosal width, and LMS did not change significantly following TL. The LMS exhibited stability throughout time, remaining the LMS below an incidental level. In addition, there was no association between the degree of sinus opacification and the preoperative LMS in both the TL group and the control group. ITSTV showed a significant decrease following TL, and we detected a significant increase in ITSTV in the 6-month postoperative period, followed by a gradual decrease up to 3 years after TL, which did not show such significant changes on the ITSTV in the control group, as represented in Figure 4. The significant impact of our study is supported by a longer follow-up time interval following TL evaluation of changes in the sinonasal structures, which is a previously unpresented finding, to the best of the author's knowledge.
In the 1990s and 2000s, many scholars studied the effect of nasal airflow discontinuation using the TL patient models. However, previous publications reported only microscopic structural changes, nasal cavity dimension using acoustic rhinometry, or mucociliary clearance following TL.7-9, 19-24 As far as the author's knowledge, the current study is the first study to report the macro-structural changes in the sinonasal structures, providing the anthropometric measurements of the sinonasal structures following TL.
On the other hand, surveillance CT image aids in, not only the assessment of the cancer recurrence, but also provides a valuable opportunity for the analysis of the paranasal sinuses and the sinonasal structures following nasal airflow cessation in TL patients, since the majority of patients undergoing TL are due to malignant neoplasm in the laryngopharyngeal region. Nevertheless, only two literature have been published so far in 2018 and 2019, using the surveillance CT scan in TL patients.11, 15 Moreover, both previous literature focused only on the changes in the severity of CRS in TL patients with a mean follow-up period less than 3 years, lacking the information on the sinonasal structural changes following TL. Our study not only provides the sinonasal structural changes following TL, but also reports the changes in each sinus opacity following TL in a longer surveillance period than the previous studies using CT images.
In a surgically closed nostril rat model, the increase in the goblet cells and ciliated cells took place on the side of obliterated nasal cavity.3, 25 Similarly, in patients who underwent TL, an increase in the goblet cells23, 26 and ciliated epithelium7, 20 were reported especially in the anterior third of the nasal cavity mucosa, with improvement in the cilia function as well.3, 7, 9, 20, 26, 27 However, these changes were noticed in the samples obtained from the inferior turbinate mucosa samples.9, 23 On the contrary, the maxillary sinus mucosa of the TL patient model showed degeneration of goblet cells.24 These previous literature suggests the nasal mucosa is directly influenced by direct exposure to the airflow, and the effect of nasal airflow cessation is thought to mainly take place in the nasal mucosa located mainly on the inferior and anterior portion of the nasal cavity, as suggested by Boyce and Eccles.25 Our study results go parallel with the previous literatures, as our data shows changes in the ITSTV, but not the paranasal sinus volume nor MSNO mucosal width.
Nonetheless, whether hypertrophic or atrophic changes occur in the nasal mucosa during the long-term follow-up after TL is controversial. In summary of previous literatures, it can be suggested that IT mucosa of TL patients undergoes a congestive-hypersecretory phase with increased ciliary cells and their activity due to increased mucus production, which gradually decreases with degeneration of glands and mucosal atrophy from 6 months up to 3 years following TL.8, 10, 23, 28, 29 Interestingly, the authors have detected a significant ITSTV increase in the 6 months postoperatively, which significantly decreased up to 3 postoperative years, and no changes after 3 years were observed (Figure 3). Increased ITSTV in the early 6 months postoperatively may have resulted from hyperplasia of goblet cells, basal lamina, congestion, and increased mucus production, followed by a substantial decrease with degenerative and atrophic changes, as suggested in previous literatures.23, 28 In addition, the authors would like to suggest that the opposed results in previous publications might have resulted from the diverse distribution of postoperative duration of study samples.
The results of our study indicate that significant changes occur in the sinonasal microstructures of the inferior turbinate soft tissue after nasal airflow ceases. The ITSTV exhibits a significant increase during the initial 6 months subsequent to the cessation of nasal airflow. Subsequently, the ITSTV undergoes a gradual decline over subsequent years, ultimately reaching a point showing a lower ITSTV value than the ITSTV prior to nasal airflow cessation. On the other hand, discontinuation of nasal airflow had no impact on the size of the maxillary sinus ostium or the volume of four paranasal sinuses. These results were exclusively observed in the TL group and not in the control group, indicating that the cessation of nasal ventilation is likely to have a greater effect on the soft tissue of the inferior turbinate than on any other sinonasal structure. On the reverse, this may thoughtfully prompt an inquiry into whether nasal breathing itself could affect the soft tissue of the inferior turbinate, and if so, whether cessation of nasal airflow could have a causative or a protective effect on the inferior turbinate and other sinonasal structures, when in real clinical practice. It is noteworthy that some previous publications have hypothesized that an increase in nasal patency and nasal airflow could serve as a causative factor, and in the development of empty nose syndrome (ENS), whose inferior turbinate has been too much resected, paradoxically causing severe difficulties in nasal breathing and nasal dryness symptoms.30, 31 In ENS patients, some reports suggest that reconstructing the structures of the inferior turbinate thus minimizing the enlargement of the nasal airway,32, 33 and even performing a transient surgical closure of the affected nostril could be effective measures to alleviate the adverse alterations observed in the nasal mucosa of patients with atrophic rhinitis.34 Therefore, it is possible to carefully hypothesize that the inferior turbinate experiences a “proliferative and congestive” phase for the initial 6 months, succeeded by a progressive “reduction and atrophic” phase, as time passes within a nasal cavity devoid of nasal respiration. Also, when considering the inferior turbinate is a main organ to be involved in the pathophysiology of allergic rhinitis (AR),35 which the inferior turbinate mucosa shows congestion and edema upon exposure to aeroallergens during nasal breathing,35 it can be also hypothesized that the exclusive changes in ITSTV following cessation of nasal airflow might be as a result of cessation in aeroallergen inhalation to the nasal cavity. However, due to the retrospective study design, we were not able to acquire any data on whether the study subjects had symptoms prior and after nasal airflow cessation, regarding on nasal symptoms, AR-specific symptoms, and the presence of atopy to specific aeroallergens, and we were also not able to obtain a serial nasal endoscopy following TL. Therefore, we suggest a further research, to clarify this matter.
TL patients are reported to have abundant bacterial colonization without inflammatory cells.21-23 To the authors' knowledge, only three previous literature have studied the CRS in TL patients.11, 15, 36 Sesterhenn et al. have reported a significant decrease in number of patients complaining of common cold and sinusitis symptoms after TL compared with the preoperative period using a questionnaire.36 In 2018, Kidwai et al. reported that the LMS did not significantly change after TL in 42 patients.11 In 2019, Patel et al. reported minimal LMS changes after TL in 50 patients, additionally suggesting that patients with preoperative CRS might develop a worsened, clinically significant CRS in the postoperative period.15 However, both studies lack a long-term time interval following TL, and both studies did not include a comparison with a control group. In the present study, a control group with similar disease burden and intervention was included. In both TL and control groups, preoperative LMS was not significantly changed after TL, and there were no significant differences in the number of patients with LMS change between the TL and control group (Table 2). Moreover, the authors have found an inconsistency in the LMS with elapsed time after TL, regardless of preoperative LMS (Figures S1 and S2). However, the authors cannot clearly define the changes in LMS over time following TL. Therefore, we propose a further study to determine the factors related to the aggravation and alleviation of CRS in TL patients in the future.
Despite the merits of the present study, the authors acknowledge some limitations due to the retrospective cohort design of the study. The severity of CRS was only assessed with LMS on CT image. As suggested by Ashraf et al., LMS <5 may be incidentally noticed in the asymptomatic, general population.18 Although there were no subjects who underwent functional sinus surgery in the surveillance period following laryngeal surgery, the lack of symptomatic assessment or endoscopic examination limits the results of our study. Second, we were not able to provide information on the volume or thickness changes in the paranasal sinus mucosa following TL. The inner mucosal lining of the paranasal sinuses shown on the CT images was too thin for accurate tracing, technically limiting the volume and thickness evaluation of the sinus mucosa. To overcome this technical limitation, we propose an evaluation using high-resolution CT or magnetic resonance images for studies in the future. Third, although we have excluded patients who required a tracheostomy more than 1-month following partial laryngectomy or hypopharyngectomy in the control group, 21 out of 38 (55.3%) had an interim tracheotomy immediately following surgery for airway protection, which the mean duration of until decannulation was 16.6 ± 2 days following surgery. Thus, although we have set the control group as having a similar disease burden and treatment course to the TL group, it must be disclosed as a limitation that our control group had undergone a very short period of nasal airflow cessation due to an interim tracheotomy immediately following surgery, which could have possibly affected the outcomes in our investigation. Lastly, we disclose and acknowledge the possible confounding that might have affected the outcomes of our study. A history of pre- or post-radiation or chemotherapy treatments was present in less than one-third of the subjects in both groups. In addition, although statistically insignificant, the TL group contained a marginally greater proportion of patients who underwent radiation therapy or chemotherapy after surgery. The detrimental effects of chemotherapy and radiotherapy on nasal ciliary clearance and sinus function, along with alterations in the sinonasal mucosa ranging from congestion to atrophic changes, have been documented in numerous recent publications, as this may have possibly been one of the confounding factors in our study.37-40 However, considering that the main reason for TL is mostly due to advanced laryngopharyngeal cancers, the effects of additional chemoradiation on the sinonasal mucosa and microstructures should be considered as the major limitation. To overcome this factor, a subgroup analysis regarding on the adjuvant chemo- or radiation status might be fruitful. However, we were unable to perform a subgroup analysis owing to a small sample size. Moreover, few evidence also suggest that age,41, 42 tobacco smoking,43 and malnutrition44-46 may also adversely influence the sinonasal mucosa. Although no significant differences in age, gender, and nutritional status were noticed in our study, it should be disclosed that the present study enrolled middle-aged, advanced laryngopharyngeal male cancer patients, and the majority of the subjects had a history of tobacco smoking in both groups, thereby limiting the applicability to subjects of more diverse age and gender, non-smoking population with no disease burden.
5 CONCLUSION
The results of this present study conclude that the paranasal sinus structures and CRS are not significantly affected, whereas the soft tissue volume of the inferior turbinate is affected by long-term cessation of nasal airflow in patients who have undergone TL.
ACKNOWLEDGMENTS
None.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.




