Treatment outcome and prognostic factors of inverted papilloma involving the frontal sinus
This paper had been Presented at the The AAO-HNSF 2022 Annual Meeting & OTO Experience on September 10–14, 2022 in Philadelphia, Pennsylvania.
Abstract
Objectives
This study aimed to evaluate the characteristics and treatment outcomes of inverted papillomas involving the frontal sinus.
Methods
Patients treated for inverted papilloma involving the frontal sinus between 2003 and 2020 were reviewed. Tumors were classified based on their extent (Extent 1: partially encroaching on the frontal sinus; Extent 2: completely filling the frontal sinus; Extent 3: eroding bony borders beyond the frontal sinus) and site of origin (Origin 1: originating outside the frontal sinus and prolapsing into the frontal sinus; Origin 2: originating from the frontal sinus walls medial to the vertical plane of the lamina papyracea; Origin 3: originating from the frontal sinus walls lateral to the vertical plane of the lamina papyracea). Treatment outcomes including tumor recurrence and patency of the frontal recess were analyzed according to tumor characteristics and surgical treatment modalities.
Results
A total of 49 surgical cases were analyzed. Extent 1 were the most common type (n = 27), followed by Extent 2 (n = 15), and Extent 3 (n = 7). The most common sites of origin were Origin 1 (n = 23), followed by Origin 2 (n = 15), and Origin 3 (n = 11). Overall, there were nine recurrences (18.4%). Recurrence was not associated with tumor extent, whereas tumor origin, particularly Origin 3 was associated with higher recurrence; 1/23 (4.3%) for Origin 1, 3/15 (20.0%) for Origin 2, and 5/11 (45.5%) for Origin 3 (Log-rank p < .001). Draf III frontal sinusotomy was associated with in the highest patency rate (84.6%) during the follow-up.
Conclusion
The recurrence rate of frontal sinus inverted papilloma depends on tumor origin rather than the extent of the tumor. In particular, lesions originating from the frontal sinus lateral to the lamina papyracea recur frequently. Draf III frontal sinusotomy can achieve patent frontal recess allowing active surveillance.
Level of Evidence
IV
1 INTRODUCTION
Sinonasal inverted papilloma is a benign tumor originating from the Schneiderian membrane of the sinonasal mucosa.1 The incidence of inverted papilloma ranges from 0.5% to 4% of primary nasal tumors.2 Although inverted papilloma is considered to be a benign lesion, compression of the adjacent neurovascular structures can occur if untreated and association with malignant lesions cannot be ignored.3 Therefore, the treatment goal is complete removal of the lesion with proper removal of the tumor origin to reduce the risk of recurrence. In most cases, this can be achieved through an endoscopic endonasal approach, however, an open craniofacial approach can be necessary depending on the location of the tumor.4
Among cases of inverted papilloma, those involving the frontal sinus are rare and account for 2.5%–6.5% of all sinonasal inverted papillomas.1, 5-7 The frontal sinus is a difficult anatomical site for surgeons not only because it is close to critical structures such as the anterior skull base and orbit, but also because of ergonomic considerations as the surgeon is working at a vertical angle. For tumors arising in the frontal sinus, complete removal is more challenging because of mucosal invagination into small vascular pits within of the sinus bone.8 For these reason, recurrence rate of inverted papilloma involving the frontal sinus is 22.4%, which is higher than that of inverted papilloma involving other sites,9 making this disease very challenging to manage.
To date, there have been few studies regarding the treatment outcomes of frontal inverted papilloma. The purpose of this study was to evaluate the characteristics and treatment outcomes of inverted papillomas involving the frontal sinus. In particular, we focused on treatment outcomes according to tumor extent, surgical approach, and tumor origin.
2 MATERIALS AND METHODS
We performed a retrospective analysis of patients with inverted papilloma with involvement of frontal sinus treated at two tertiary hospitals between 2003 and 2020. Patients with a history of tumor removal were regarded as revision cases. Patients with associated malignancy and sinonasal papilloma other than Schneiderian papilloma were excluded from the study. Tumor characteristics, revision status, surgical approach, postoperative frontal recess patency, and recurrence were analyzed.
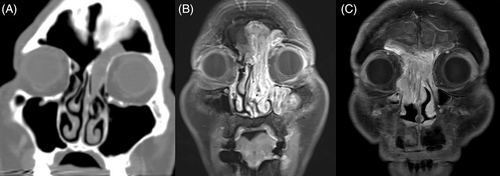
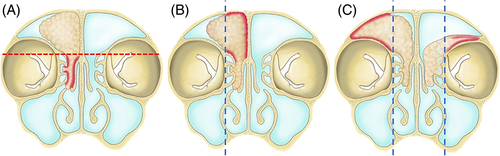
Tumors were first classified based on their extent and site of origin. Extent 1 referred to a tumor that partially filled the frontal sinus, Extent 2 referred to a tumor that completely filled the entire frontal sinus, and Extent 3 referred to a tumor that eroded the bony borders beyond the frontal sinus and extend to adjacent structures such as the frontal sinus posterior wall, lateral lamella, orbit wall, or contralateral side (Figure 1). According to the origin of the tumor, Origin 1 referred to a tumor originating outside the frontal sinus and prolapsing into the frontal sinus. The criterion for dividing the inside and outside of the frontal sinus was the frontal beak anteriorly and the most anterior part of the anterior skull base posteriorly. Origin 2 referred to a tumor originating from the frontal sinus walls medial to the vertical plane of the lamina papyracea. Finally, Origin 3 referred to a tumor originating from the frontal sinus walls, lateral and superior to the vertical plane of the lamina papyracea (Figure 2).10 Therefore, Origin 3 includes possible origin sites such as the lateral or superior frontal sinus or frontal recess cells situated on the lateral side of the vertical plane of the lamina papyracea, such as supraorbital cells. Origin 3 also encompasses situations with a multifocal origin, one of which is situated laterally in the vertical plane of the lamina papyracea, as well as cases where there is a diffuse origin throughout the frontal sinus. The extent and origin of the tumor were determined by a comprehensive analysis of preoperative imaging and surgical records based on intra-operative assessment. All the patients underwent contrast-enhanced computed tomography (CT). Lesions that filled the frontal sinus showing contrast enhancement were considered tumors.11 The hyperostosis or cerebriform pattern was analyzed to determine the tumor origin.12 When available, the patient underwent magnetic resonance imaging (MRI) with contrast enhancement for better assessment. The goal of the surgery was gross total resection. Surgical approaches were determined by the pre- and intra-operative surgical findings and were classified as follows13, 14: (1) Draf IIA, (2) Draf IIB, (3) Draf III, and (4) endonasal approach in combination with various external approaches, including trephination, miniosteoplastic flap,15 and standard osteoplastic flap surgery. Orbital transposition was also performed to removal the lesion located laterally when necessary.16 After gross total resection, manipulation of the tumor origins was described as electrocauterized or drilled (with or without electrocauterization) (Figure 3). For the patients with missing information for the treatment of tumor attachment site at the operation record, it was described as N/A.
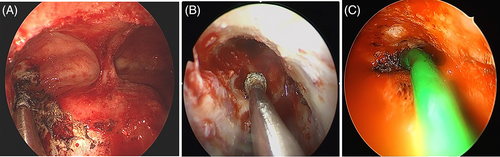
The usual post-operative follow-up visit schedule is as follows: every 3 months for the first year, every 6 months in the second year, and yearly afterwards. Routine endoscopic examinations were conducted during each visit. When there was a suspicious lesion or if the frontal recess was not patent enough for adequate visualization, CT or MRI examinations were also conducted. Recurrence was identified by routine endoscopic examination, contrast CT or MRI, followed by pathologic confirmation. The patency of the frontal recess was evaluated by endoscopic examination and was regarded as patent if it was more than 50% of the size of the frontal recess developed at the end of the previous surgery.17 As the dimensions of the frontal recess may change during the wound healing process, patients who had been followed up for less than 3 months were excluded when analyzing factors related to the frontal recess patency.
All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA). Statistical significance was set at p < .05. Survival analysis was performed using Kaplan–Meier estimates and Cox regression analyses (forward selection method). Recurrence-free survival was measured until the date of the first diagnosis of recurrence or, if censored, the date of the last follow-up for patients without evidence of residual disease. Categorical variables are presented as numbers, and continuous variables are presented as mean ± standard deviation. Linear by linear association chi square test analyses were used to evaluate the association between the factors. This study was approved by the Institutional Review Board of the Seoul National University Hospital (Seoul, Korea; IRB No. H-2203-088-1308) and Seoul National University Bundang Hospital (Seongnam, Korea; IRB No. B-2212-799-401).
3 RESULTS
3.1 Patient characteristics
A total 49 patients were enrolled from 2003 to 2020. Eighteen patients had a history of tumor removal outside our institution and were regarded as revision cases. Three patients exhibited dysplasia upon histopathologic examination. The mean age of the patients was 60.6 ± 16.2 years. The male: female ratio was 34:15. According to the extent of the disease, 27 patients were classified as Extent 1 (55.1%), 15 as Extent 2 (30.6%), and the other 7 as Extent 3 (14.3%). According to tumor origin, 23 were classified as Origin 1 (46.9%), 15 as Origin 2 (30.6%), and the other 11 as Origin 3 (22.4%) tumors. The surgical approaches included 20 (40.8%) Draf IIA, 13 (26.5%) Draf IIB, 11 (22.4%) Draf III, and 5 (10.2%) external combined approaches. Orbital transposition was performed in three cases. For tumor attachment sites, 19 patients (38.8%) were electrocauterized, 15 patients (30.6%) were drilled, and the remaining 15 patients (30.6%) were marked as N/A. Tables 1 and 2 summarize the clinical characteristics of the patients and the detailed surgical approaches.
| Total (N = 49) | |
|---|---|
| Age (year) | 60.6 ± 16.2 |
| Sex (M:F) | 34:15 |
| Number of revision case (%) | 18 (36.7%) |
| Dysplasia (%) | 3 (6.1%) |
| Surgical approach (N) | |
| Draf IIA | 20 (40.8%) |
| Draf IIB | 13 (26.5%) |
| Draf III | 11 (22.4%) |
| External combined | 5 (10.2%) |
| Orbital transposition (%) | 3 (6.1%) |
| Tumor origin (N) | |
| Origin 1 | 23 (46.9%) |
| Origin 2 | 15 (30.6%) |
| Origin 3 | 11 (22.4%) |
| Tumor extent (N) | |
| Extent 1 | 27 (55.1%) |
| Extent 2 | 15 (30.6%) |
| Extent 3 | 7 (14.3%) |
| Management of tumor origin (N) | |
| Electrocautery | 19 (38.8%) |
| Drilling (with or without electrocautery) | 15 (30.6%) |
| N/A | 15 (30.6%) |
| Patient | Revision state | Sex/Age | Extent | Origin | Endonasal endoscopic approaches | External combined approaches |
|---|---|---|---|---|---|---|
| 1 | Yes | F/52 | Extent 3 | Origin 2 | Draf IIA | Mini osteoplastic flap |
| 2 | Yes | M/75 | Extent 2 | Origin 3 | Draf III | Osteoplastic flap |
| 3 | Yes | F/88 | Extent 2 | Origin 3 | Draf III | Trephination |
| 4 | No | F/67 | Extent 2 | Origin 3 | Draf IIB | Trephination |
| 5 | No | M/81 | Extent 2 | Origin 3 | Draf IIB | Trephination |
3.2 The associations between tumor characteristics and surgical approaches
The associations between tumor characteristics and surgical approaches are summarized in Table 3. There was a significant association between the tumor extent and surgical approach (p = .010). The majority of the tumors that did not extend beyond the frontal sinus were usually managed by Draf IIA and Draf IIB (77.7% for Extent 1, 66.7% for Extent 2). However, for Extent 3, which extends the frontal sinus into adjacent structures, 71.4% were managed using Draf III or combined external approaches. There was also a significant association between the origin of the tumor and the surgical approach; Origin 3 was associated with a more extended type of surgery (Draf III or combined external approach), while Origin 1 was associated with a less extended surgery (mostly Draf IIA; p < .001). There was no significant association between ablation techniques for the tumor attachment site and the location of the tumor origin (p = .077). No further analysis of the techniques used for tumor origin was conducted as N/A constitutes about 30%.
| Draf IIA | Draf IIB | Draf III | External combined | p-Value | ||
|---|---|---|---|---|---|---|
| Tumor origin (N) | Origin 1 | 16 | 6 | 1 | 0 | <.001 |
| Origin 2 | 3 | 6 | 5 | 1 | ||
| Origin 3 | 1 | 1 | 5 | 4 | ||
| Tumor extent (N) | Extent 1 | 13 | 8 | 6 | 0 | .010 |
| Extent 2 | 7 | 3 | 1 | 4 | ||
| Extent 3 | 0 | 2 | 4 | 1 |
| Origin site ablation technique | Electrocautery | Drill | p-Value | |
|---|---|---|---|---|
| Tumor origin | Origin 1 | 11 | 3 | .077 |
| Origin 2 | 4 | 7 | ||
| Origin 3 | 4 | 5 | ||
3.3 Treatment outcome
Of the 49 patients, 9 (18.4%) showed evidence of recurrence. The median recurrence-free interval was 13.0 months and the 3-year recurrence free survival rate was 75.8%. There was no significant difference in recurrence-free survival according to tumor extent, revision status, or surgical approach (log-rank p = .411, .661, and .288, respectively; Figure 4A–C). However, recurrence-free survival was significantly different according to the tumor origin site (log-rank p < .001; Figure 4D). Overall, the recurrence rate was 1/23 (4.3%) for Origin 1, 3/15 (20.0%) for Origin 2, and 5/11 (45.5%) for Origin 3 tumors.
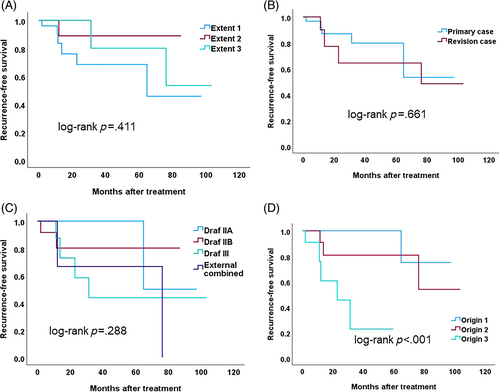
A multivariable Cox regression analysis was performed to evaluate factors related to recurrence-free survival. The factors included tumor extent, revision status, tumor origin, and surgical approaches. All patients presenting with dysplasia (n = 3) or undergoing orbital transposition (n = 3) did not experience recurrence. Because of their low incidence compared to other parameters, these factors were not included in the analysis. Only the origin of the tumor was associated with higher disease recurrence; Origin 3 had significantly higher risks of recurrence compared to Origin 1 (hazard ratio = 25.9, 95% confidence interval = 2.5–268.0).
The relationship between frontal recess patency and the type of surgery was examined (Table 4). Only the endonasal approaches (e.g., Draf IIA, Draf IIB, or Draf III) were evaluated in patients who underwent combined external approaches. Four patients with patent frontal recesses were excluded as they were followed up for less than 3 months. Frontal recess patency was achieved in 61.1% (11/18), 61.5% (8/13), and 84.6% (11/13) of Draf IIA, Draf IIB, and Draf III patients, respectively (p = .205). The extended endonasal approach to the frontal sinus appeared to exhibit a higher patency rate; however, this difference did not achieve statistical significance. Nevertheless, among Origin 2, the patency rates for Draf IIA, Draf IIB, and Draf III were 25.0%, 60.0%, and 100.0%, respectively (p = .024). These results suggest an increased patency rate associated with the more extended endonasal approach, particularly for cases with tumor Origin 2.
| Patent | Stenosis | p-Value | ||
|---|---|---|---|---|
| All (N = 45) | Draf IIA | 11 | 8 | .133 |
| Draf IIB | 8 | 5 | ||
| Draf III | 11 | 2 | ||
| Origin 1 | Draf IIA | 9 | 5 | .361 |
| Draf IIB | 4 | 1 | ||
| Draf III | 1 | 0 | ||
| Origin 2 | Draf IIA | 1 | 3 | .024 |
| Draf IIB | 3 | 2 | ||
| Draf III | 5 | 0 | ||
| Origin 3 | Draf IIA | 1 | 0 | .868 |
| Draf IIB | 1 | 2 | ||
| Draf III | 5 | 2 |
4 DISCUSSION
In the current study, we reviewed our cohort of patients who had been treated for inverted papilloma involving the frontal sinus. Overall recurrence occurred in 18.8% of patients, which is comparable to other studies.9, 10 Among clinical factors, including tumor extent, tumor origin, and revision status, recurrence was only associated with the tumor origin site. Especially, the recurrence rate for patients with tumors originating from the lateral lamina papyracea was as high as 45.5%.
The treatment principle for inverted papilloma is complete removal of the involved mucosa along with the periosteum and the bone underlying the diseased mucosa. The importance of tumor origin as a significant prognostic factor highlights the necessity for thorough removal of the origin. This is particularly crucial given that Origin 3 poses the greatest challenge in terms of accessibility compared to the others.
Recent advancements in endoscopic sinus surgery allow us to surgically remove frontal sinus inverted papillomas in most cases.4, 18 Draf IIA or IIB may be sufficient when only the origin is in the outer or medial side of the frontal recess. When the tumor origin is located laterally, more extended surgical approaches to the frontal sinus are necessary. Endoscopic Draf III provides additional access to the lateral side of the frontal sinus as the angle of the attack increases with removal of the inter-frontal sinus septum and approaches from the contralateral side of the nostril.19 Endoscopic orbital transposition procedure, which may provide additional access by lateralizing the orbital content.16 However, for tumors originating from the far lateral side, anterior, or superior wall of the frontal sinus, or those involving diffuse mucosa on the frontal sinus, an additional external approaches is necessary. In our study, a higher degree of tumor extension into the frontal sinus (Origin 1 or Extent 1) corresponded with more extensive surgical approaches, including external approaches. This correlation aligns with the nuances of surgery in frontal sinus inverted papilloma.
Although frontal sinus trephination could be an alternative option in such circumstances, visualization and manipulation are difficult, as these should be performed through a small port that is not ergonomically feasible. In a study of 47 cases with frontal sinus inverted papilloma by Pietrobon et al., 61.7% were managed by endoscopic endonasal approach in combination with osteoplastic flap surgery.20 They demonstrated only two recurrent cases which is lower than our study. Therefore, when surgeons are not sure about complete removal with endoscopic techniques, external combined approaches such as a standard osteoplastic flap technique may be required.4 However, our results did not demonstrate a reduction in relapse when using external approaches. This seems to have resulted from the small number of patients in our study, and therefore, further research is needed.
In the current study, tumors that completely filled the frontal sinus could be managed by Draf IIA or Draf IIB. However, even tumors that completely filled the frontal sinus did not exhibit a higher risk of recurrence compared to those partially filling the frontal sinus. Traditionally, it is recommended that osteoplastic flap surgery should be performed in patients with extensive frontal sinus involvement.21 However, if the mass is occupying the frontal sinus without mucosal involvement, the tumor can be removed without stripping all of the adjacent mucosa, and therefore the tumor can be simply pulled out without necessitating extended frontal sinus approaches.20, 22
It seems to be obvious that wider exposure during the operation is beneficial for complete removal of the tumor. Therefore, there may be no disagreement that Draf III or combined external approaches are preferred surgical approaches for tumors with Origin 3. However, when dealing with Origin 2, many of the tumors can be removed using less extensive approaches (Draf IIA or Draf IIB). This raises questions about whether extended approaches (e.g. Draf III) could offer additional benefits in this scenario. Considering the patency of the frontal recess, Draf III seemed to maintain a highest patency rate. Stripping off the diseased mucosa along with the periosteum and removal of the adjacent bone during limited frontal sinusotomy may have resulted in a higher rate of developing frontal recess stenosis.23 Therefore, when choosing an approach, the accessibility of the frontal sinus to eradicate the lesion and maintenance of frontal recess patency should be considered.
Few studies have described the outcomes of frontal sinus inverted papilloma treatment. To the best of our knowledge, this is the first study to reveal factors associated with treatment outcomes, including recurrence and maintenance of the frontal recess. However, our study had several limitations. First, external approaches such as trephination, mini-osteoplastic flap, and standard osteoplastic flap were not analyzed separately because of the small number of patients. Second, the origin of the tumor needs to be further specified as the anterior wall and the superior portion of the frontal sinus is more difficult to access than the lower and posterior walls. In addition to the anatomical location of the tumor origin, other characteristics such as diffuse, multifocality (synchronicity), or localized tumor origins were not distinguished. Since there is no system to describe the side of attachment, there were difficulties in accurately describing the exact location of the tumor. This, in turn, adds to the challenges in conducting retrospective analyses. Third, dysplasia is recognized as an important prognostic factor.24 However, due to the limited number of patients, it was not considered as an analytical factor in the present study. Fourth, although gross total resection was achieved in all patients, ablation techniques for the tumor attachment site, which could further impact treatment outcomes, have not yet been evaluated completely. Some cases were missing, rendering further statistical analysis potentially meaningless. In addition, the techniques may depend on various factors, such as anatomical location, the availability of surgical instruments (e.g., drills at the time of surgery), and the surgeon's preference. It is noteworthy that a recent meta-analysis demonstrated no significant association between the techniques utilized for eradication and the recurrence rate of IP and yet, certain techniques were favored depending on the IP attachment site.25 Lastly, when evaluating the patency of the frontal recess, procedures which may affect the outcome such as mucosal flap coverage over the frontal recess had not been considered.26 Due to the limitations resulting from the factors mentioned above, in the future a large-scale multicenter study is warranted. Future research should also address the algorithmic approach for selecting the best surgical approach after considering the tumor origin, recurrence, and patency of the frontal recess.
5 CONCLUSIONS
The overall recurrence rate was 18.4%, and tumor origin was the most important factor associated with recurrence rate. Although tumors located lateral to the lamina papyracea can usually be managed by extended frontal sinus surgery, recurrence was significantly higher. An endonasal Draf III frontal sinusotomy, especially for tumors origins located in the true frontal sinuses may provide higher patency of the frontal recess, allowing for active surveillance.
FUNDING INFORMATION
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) [grant number NRF-2022R1F1A1076298].
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.




