How Did the COVID-19 Pandemic Change Cigarette Smoking Behavior?
Funding: The author received no specific funding for this work.
ABSTRACT
Background
Cigarette smoking is the leading cause of preventable death in the United States. Although smoking rates have been declining, it is unclear how the COVID-19 pandemic impacted smoking behaviors.
Methods
With population-based data from the Behavioral Risk Factor Surveillance System (2010–2023), we will analyze four outcomes: (1) currently smoking, (2) started smoking in the past year, (3) number of cigarettes per day, and (4) quit smoking in the past year. By estimating a two-way fixed effect regression model to account for state-level factors and temporal trends, this study identifies the effect of exposure to the COVID-19 pandemic by comparing the change over time in an unexposed group (interviewed January 1–March 20, 2020) to the change over time in the exposed group (interviewed January 1–March 31, 2021–2023). This stage 1-registered report conducts a Power and pilot analysis with an exploratory outcome: being uninsured.
Pilot Results
Our power analysis calculates a minimum detectable effect size = 0.7%. The pilot analysis indicates that exposure to the COVID-19 pandemic was associated with a statistically significant decline in the probability of being uninsured (Est. = −2.2%; CI = −3.5, −1.1). There is little evidence that the early and late survey wave cohorts differed before the pandemic.
Implications
This registered report outlines a study aimed at investigating the direct impact of the COVID-19 pandemic on smoking behaviors related to prevalence, initiation, intensity, and cessation. The findings will provide valuable insights into the effects of public health crises on health-related behaviors and inform future public health interventions. The preregistration of the study design and analysis plan ensures transparency, trust, and replicability of the results. Quantifying whether and how smoking behaviors changed, and in whom, can inform ongoing tobacco control efforts to continue the downward trend in cigarette smoking.
Preregistration
1 Introduction
For decades, cigarette smoking has been the leading cause of preventable death in the United States [1, 2]. Compared to nonsmokers, adults with a history of smoking are more likely to develop and die from heart disease, stroke, and various cancers (including the leading cause of cancer mortality: lung cancer) [2-5]. In 2019, 10% of all adults reported smoking cigarettes, with elevated prevalence for adults experiencing socioeconomic vulnerabilities and poor health outcomes [6, 7]. Then, in 2020, smoking was found to be associated with increased risk of death during the COVID-19 Public Health Emergency [8-10].
While the relationship between smoking and adverse COVID-19 outcomes was clear early in the pandemic, there was less certainty about how smoking behavior would change during the pandemic. Leading up to March 2020, smoking rates have been declining [6, 11, 12]. Perhaps smoking rates would continue to decline as a response to the elevated risk of COVID-19 for smokers or the loss of income from the economic downturn [13]. Or, conversely, smoking rates would rise during the pandemic, given the relationship between smoking and social stressors, isolation, and unemployment [14-17]. In addition to potential changing prevalence, the intensity of smoking could also change (i.e., more or less number of cigarettes per day during the pandemic). Finally, quitting rates or attempts could also change because of health system disruptions. This effect, too, however remains ambiguous. While smoking cessation participation could decline early in the pandemic due to canceling or delaying elective healthcare services [18], the advent of tele-smoking cessation could lead to greater participation in the longer term [19].
The limited evidence examining smoking trends during the pandemic has been mixed. On a global scale, there was no consensus on whether or how smoking changed during the pandemic [20]. Research in the United States, which mostly used convenience samples at a single time-point [20], showed that cigarette smoking increased 1–2% points for college students and metropolitan adults experiencing lockdowns or social stress [21-23]. However, a subsequent study showed that job loss during 2020 was associated with reduced smoking, regardless of unemployment insurance status [24]. From the few population-based studies, longitudinal survey analyses found that smoking rates in older adults did not change during the pandemic, even when considering mechanisms such as loneliness, depression, and social isolation [25]. The largest population-based study found that smoking prevalence declined 1–2% points among most of the population, with the largest reductions among those temporarily unemployed [26].
1.1 Objective
Using the latest, population-based survey data (released October 2023) [27], this study aims to evaluate how smoking behaviors changed in years 1, 2, and 3 of the COVID-19 Public Health Emergency. The limited high-quality, population-based evidence suggests that smoking prevalence declined in 2020, which conflicts with the nonrandom, point-in-time studies. The existing evidence also fails to illuminate how the pandemic may have impacted the intensity of smoking and cessation rates, as well as how general smoking trends may have changed over the course of the pandemic. Failure to understand how smoking behaviors changed risks reversing major gains in tobacco prevention and control. This study also aims to explore heterogeneity by sociodemographic factors: age, sex, race/ethnicity, education status, marital status, and region. Finally, given the dynamic trends in tobacco use more generally (rising electronic cigarette use amid declining smoke and smokeless tobacco use), we are among the first to assess how these other types of nicotine and tobacco trends changed during the COVID-19 pandemic.
1.2 Hypotheses
-
The prevalence of current smokers changed during the COVID-19 pandemic.
-
The prevalence of adults who initiated smoking changed during the COVID-19 pandemic.
-
The average number of cigarettes (packs) smoked per day changed during the COVID-19 pandemic.
-
The prevalence of adults who quit smoking changed during the COVID-19 pandemic.
Each of the four hypotheses will be tested using a two-sided test of statistical significance. Additionally, each of the four hypotheses will also test if the respective change differed between years 2020, 2021, and 2022. Finally, each of the four hypotheses will test if the respective change differed within sociodemographic subgroups (sex, age, race/ethnicity, education status, marital status, census region).
2 Methods
2.1 Data
Data for this study comes from the Behavioral Risk Factor Surveillance System (BRFSS). BRFSS is a nationally representative, population-based survey conducted annually in the United States. Considered the gold-standard public health surveillance data, BRFSS collects self-reported data on various health-related behaviors, chronic health conditions, and access to healthcare services from a representative sample of the noninstitutionalized U.S. population. The 2022 release of the BRFSS dataset is the most recent data available, providing us with the most up-to-date insights into the health behaviors and conditions of the U.S. population. Conducted by phone, the response rates were less affected by the pandemic than other nationally representative surveys. BRFSS has been a commonly used dataset for assessing how healthcare services and behaviors changed during the COVID-19 pandemic [26, 28–33].
In addition to smoking behavior responses, BRFSS collects sociodemographic and geographic data (state). The Centers for Disease Control (CDC) uses a complex procedure to select a random, representative sample of each state in the United States. This study includes only respondents from the 50 states and Washington, D.C. The CDC implements the BRFSS questionnaire in waves, interviewing participants each month of the year. Whether a person is interviewed in 1 month or another, is effectively random based on sampling design. Each BRFSS survey wave spans 15 months from January 1 of the initial year to March 31 of the following year. For the purposes of this study, all responses between April 1 to December 31 will be excluded.
2.2 Outcomes
2.2.1 Primary Outcomes
The first primary outcome is a binary variable indicating if the respondent currently smokes, either every day or some days. In 2022, 12% of the respondents answered “every day” or “some days” to the question “Do you now smoke cigarettes every day, some days, or not at all?”
So, if individual i is 30 and started smoking at age 28 and the survey year t is 2022, that individual's STARTED variable will equal 1. Prevalence of this data is pending access and analysis of the data. The BRFSS codebook for 2022 reports that 153,326 adults reported initiating smoking between ages 1 and 100.
To assess the intensity of smoking, we will create a continuous variable derived from the question “On average, when you smoke regularly, how many cigarettes do you usually smoke each day?” The BRFSS question is not specific to adults currently smoking, so adults who are not reported to be currently smoking will have a zero for this variable. 150,246 respondents reported smoking between 1 and 300 cigarettes a day. BRFSS creates a “packs per day,” where this number is divided by 20, which we will use in conjunction with the cigarettes per day outcome.
Finally, to assess how the pandemic impacted smoking cessation, a binary variable QUIT will be derived from the categorical question “How long has it been since you last smoked a cigarette, even one or two puffs?” This variable will equal 1 for respondents indicating that they last smoked within the past 0–12 months, zero else.
2.2.2 Secondary Outcomes
The first secondary outcome is a binary variable indicating if the respondent currently uses smokeless tobacco. The second secondary outcome is a binary variable indicating if the respondent currently uses electronic cigarettes.
2.3 Exposure
Respondents will be considered exposed to COVID-19 if they were interviewed after March 2020.
2.4 Design
2.4.1 Framework
2.4.2 Identification
Our identification relies on the assumption that, in the absence of the COVID-19 pandemic, the trends between the early and late cohorts would have remained similar (parallel or common trends) [35]. We empirically test this assumption by conducting a set of pretreatment differential trend tests for each outcome.
2.4.3 Empirical Model
Here, the B′ parameters estimate the ATE or average association between exposure to COVIDmt and the outcome. Where Yimst is the outcome response of individual i, in month m, survey wave year t, and state s. We account for temporal trends with a set of survey wave year fixed effects (WAVEt) for both the late and early cohorts. We adjust for seasonal differences by including month fixed effects (MONTHm) and state-level differences with state fixed effects (STATEs). The vector represents a set of dummy variable controls, which adjust for age (5-year age groupings), sex (male), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic), marital status (married, divorced, or separated, widowed), and education status (no high school degree, high school degree only, some college but no degree).
To test for differences between the first, second, and third years of the pandemic (2020, 2021, and 2022, respectively), we will estimate each model above separately for each exposed year. For example, the pilot data (2020), excludes survey waves 2021 and 2022 and estimates the impact of the pandemic on smoking behaviors in 2020. The second model will drop the late cohort in 2020 and the early cohort in 2021 to test for the impact of exposure to the pandemic in 2021 (maintaining the early cohort 2020 as the reference group). The third model will drop the late cohort in 2021 and the early cohort in 2022 to test for the impact of exposure to the pandemic in 2022 (maintaining the early cohort 2020 as the reference group).
Here, the alpha (a′) parameters measure the differential trends between the early cohort and late cohorts in all the prepandemic years (2019 as reference). Statistically significant alpha parameters would signal a potential threat to our identification assumption and internal validity. The year-by-year trends will also be displayed visually.
2.5 Statistical Analysis
2.5.1 Primary Analysis
All models described above will be estimated by a linear model. Binary outcomes will be estimated by a linear probability model, whereas continuous variables will be estimated by normal linear regression models. All analyses will incorporate BRFSS-supplied sampling weights. Given that the BRFSS design samples by state, standard errors robust to heteroskedasticity will be clustered at the state level. In addition to effect estimates, we will report the confidence interval. All analyses will be conducted in STATA v. 18 [36].
2.5.2 Secondary Analysis
For the cigarettes per day and packs per day estimates, the primary analysis estimates the average impact of exposure to the COVID-19 pandemic. However, prior work suggests that this may misrepresent the impact by failing to consider the skewed nature of smoking intensity. To overcome this limitation, two alternative models will be estimated: (1) unconditional quantile regression models [37, 38] and (2) two-part models [39, 40].
If the pretrend tests reveal noncommon trends between early and late cohorts, we will implement a variety of empirical modifications consistent with the difference-in-differences literature. These include adding a running year variable to allow for differential slopes between early and late cohorts. We will also use year-by-state interaction fixed-effects term to account for differential changes in smoking behavior by state over time; an approach which also accounts for differential intensity of the COVID-19 pandemic in each state and year.
Finally, alternative specifications will be implemented to assess the robustness and sensitivity of the primary results. These alternative specifications will include different weighting strategies and approaches to estimate standard errors or statistical significance [41]. These results will not be used to interpret the hypotheses stated above but will be used to discuss whether our primary results were sensitive to analytic decisions.
2.5.3 Pilot Analysis
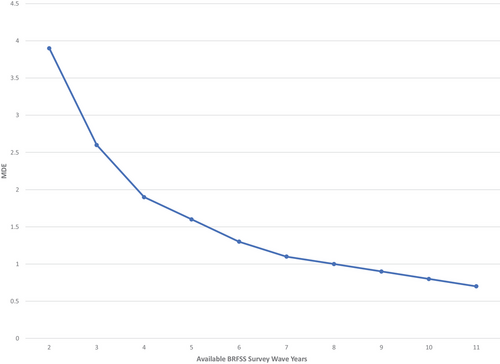
To examine the validity and analytical power of this methodology, we conducted a power analysis and pilot test with an exploratory outcome variable. In our power analysis, we calculated the minimum detectable effect (MDE) size [42]. Specifying our Power at 0.8, we calculated the MDE at the sample (panel) size of 1224, with 1137 degrees of freedom (87 fixed-effect and control variables). We also report a range of MDEs at various panel sizes, based on the number of years available for each BRFSS outcome. Second, we implemented our design and analytic model to estimate the association between exposure to COVID-19 on a pilot outcome: being uninsured at any time during the past year (Supplemental Exhibits 1–3).
2.6 Subgroups
To inform targeted interventions, we report subgroup analyses by stratifying the sample by age (below age 65, age 65+), sex (male, female), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic other race), marital status (married, not married), education (no college degree, college degree), and census region (NE, S, MW, W).
2.7 Limitations
This study is not without its limitations. First, regarding data, we chose not to include control variables which may be potentially endogenous to the COVID-19 pandemic. These include variables related to a person's ability to seek care (insurance status), health (self-rated health or chronic disease status), and employment status. Although these variables could in fact be related to changes in smoking behavior during the pandemic, our design could be biased by including a control variable which could also be affected by our exposure of interest (i.e., insurance, health, and/or employment status changes after exposure to the pandemic). Moreover, given the lack of granularity and cross-sectional nature of the data, we avoid attempting to identify any mechanisms linking exposure to the pandemic and smoking behavior changes. Regarding our design, the main limitation stems from the inability to create a valid unexposed group. First, we are assuming that respondents surveyed between January 1, 2020, to March 20, 2020, were not exposed to the COVID-19 pandemic. Given the COVID-19 pandemic and public health emergency timeline [43] as well as policy and behavioral responses, we feel this assumption is valid. However less likely, it should be noted as a potential limitation that smoking behavior could have been affected by awareness of the SARS-CoV2 spread across the globe and United States in the early months of 2020. Beyond the first few months, everyone, regardless of where they lived or the risk of adverse COVID-19 outcomes, was exposed to the pandemic and public health emergency. Therefore, we use the respondents surveyed in January 1–March 20, 2020, as the unexposed reference group for all analyses. This approach requires an assumption that the changes we observed in the late cohorts for 2020, 2021, and 2023 would be consistent with the changes for the early cohorts in years 2021 and 2023. We cannot test this assumption, empirically, without a valid unexposed group. Instead, we show the year-by-year trends for the early and late cohorts throughout the study period to assess how well they track in years two and three of the public health emergency.
3 Pilot Results
3.1 Summary Statistics
The following summary statistics were derived from the BRFSS data dictionary and codebook documentation for years 2019–2022 survey waves (Table 1). The latest sample, survey wave 2022, is estimated to include 130,527 respondents (aged 18–99). This includes 25,659 adults surveyed between January 1–March 31 of 2023 and 104,868 adults surveyed between January 1–March 31 of 2022. The response rates range from 50% in 2019 to 44% in 2021. The percentage of adults reporting to have smoked 100 cigarettes in their lifetime ranges from 40% in 2022 to 42% in 2019.
| 2019 | 2020 | 2021 | 2022 | |
|---|---|---|---|---|
| Early cohort (n) | 99,905 | 82,655 | 103,796 | 104,868 |
| Late cohort (n) | 18,650 | 12,132 | 23,508 | 25,659 |
| Response rate | 50% | 48% | 44% | 45% |
| % Smoking 100 lifetime cigarettes | 42% | 41% | 40% | 40% |
Using pilot data from BRFSS surveys 2010–2020, we found that the late cohort comprised 8% of the pre-COVID sample. Whereas, in 2020, the late cohort comprised 11% of the post-COVID sample (Table 2).
| Pre-COVID (2010–2019) | Post-COVID (2020) | |
|---|---|---|
| Early cohort | 1,014,928 | 91,662 |
| 92% | 89% | |
| Late cohort | 89,714 | 10,894 |
| 8% | 11% |
Table 3 shows the pre-COVID (2010–2019) summary statistics (mean proportions) of the exogenous, sociodemographic control variables used in the analysis for the early and late cohorts. The unadjusted table does not account for population weights, whereas the adjusted means account for population-based weighting.
| Unadjusted (%) | Adjusted (%) | |||
|---|---|---|---|---|
| Early cohort | Late cohort | Early cohort | Late cohort | |
| Male | 42.4 | 44.9 | 48.9 | 48.6 |
| Age 18–24 | 5.2 | 6.8 | 12.6 | 13.2 |
| Age 25–29 | 4.6 | 5.8 | 8.1 | 8.9 |
| Age 30–34 | 5.5 | 6.5 | 9.4 | 10.3 |
| Age 35–39 | 5.9 | 6.8 | 8.1 | 8.5 |
| Age 40–44 | 6.4 | 7.0 | 8.8 | 8.8 |
| Age 45–49 | 7.5 | 7.3 | 7.8 | 7.5 |
| Age 50–54 | 9.4 | 9.0 | 9.6 | 9.1 |
| Age 55–59 | 10.6 | 9.9 | 8.1 | 8.0 |
| Age 60–64 | 11.2 | 10.6 | 8.0 | 7.8 |
| Age 65–69 | 10.5 | 10.2 | 6.2 | 6.3 |
| Age 70–74 | 8.6 | 8.1 | 5.0 | 4.8 |
| Age 75–79 | 6.4 | 5.4 | 3.9 | 3.2 |
| Age 80–84 | 8.2 | 6.7 | 4.5 | 3.7 |
| non-Hispanic White | 78.2 | 73.2 | 65.7 | 58.1 |
| non-Hispanic Black | 7.8 | 9.4 | 11.4 | 11.0 |
| Hispanic | 6.3 | 8.9 | 13.7 | 19.6 |
| High school only | 28.6 | 27.1 | 28.5 | 27.6 |
| Some college, no degree | 27.5 | 28.1 | 30.5 | 30.4 |
| College degree | 35.9 | 37.1 | 27.5 | 27.3 |
| Married | 53.2 | 53.5 | 52.2 | 50.6 |
3.2 Power Tests
Our primary power analysis calculates an MDE = 0.007. For our binary variables, this would allow us to detect a 0.7% point change in the probability of the outcome, well within the 1–2% point changes observed in the existing evidence. Depending on the years available for each outcome, the MDE ranges from 0.7% points, when all 10 preperiod years are included in the analysis, to 3.9% points, when only one preperiod year is included in the analysis (Figure 1).
Ethics Statement
This study is not human subjects research.
Conflicts of Interest
The author declares no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is restricted by a third party. All analytic code for compiling, creating, cleaning, and analyzing the data can be found on the author's repository https://github.com/jsemprini/covid_smoking




