Evaluating aggregate stability, surface properties and disintegration behaviour of bauxite residue induced by Ca/Na
Funding information: The National Key Research and Development Program of China, Grant/Award Number: 2019YFC1803604; National Natural Science Foundation of China, Grant/Award Number: 41701587; Fundamental Research Funds for the Central Universities of Central South University, Grant/Award Number: 202045010
Abstract
Bauxite residue, a typical industrial solid waste which contains a large amount of Na+, is usually physically degraded. Understandings of aggregate formation, which is a critical process in soil development, are essential to facilitate ecological rehabilitation on the disposal areas. However, few studies have investigated the aggregation behaviour and mechanisms of key salt ions (Ca2+ and Na+) in residue aggregates. Therefore, an integrated method of Le Bissonnais' method, the combined determination method and laser diffraction measurements was applied to evaluate aggregate stability, surface properties and disintegration behaviour of bauxite residue following Ca/Na additions. With increasing Ca2+ addition, mean weight diameter increased, indicating improved resistance to dispersion. Ca2+ had a positive effect on flocculation of silt-size microaggregates, while disintegration was induced following Na+ addition. Repeated laser diffraction analysis of residue samples circulating in 50 mmol L−1 electrolyte solution (Ca2+/Na+) provided a detailed view of the changes in particle size distribution as aggregates fragmented. The visualized three-dimensional surface map revealed that Na+ promoted the disintegration of >250 μm aggregates into finer dispersed particles, while Ca2+ protected the microaggregates from fragmenting into smaller particles. Variation in electrochemical properties of aggregate surfaces affected the micro-morphology significantly. The findings provide a new approach to specify pedogenic aggregate behavior of bauxite residue, while revealing the effects of Ca2+/Na+ on aggregate stability, surface electrochemical properties and its micromorphology. The results will provide a detailed understanding of aggregate behavior during soil formation process in bauxite residue.
1 INTRODUCTION
Bauxite residue, a highly alkaline solid waste, is produced by alumina extraction from bauxite ore by the Bayer process (Cusack, Courtney, Healy, Donoghue, & Ujaczki, 2019; Santini, Kerr, & Warren, 2015). Globally, the inventory of bauxite residue has reached 4.6 Gt, and increased at a rate of 200 Mt per annum (Xue et al., 2020). Disposing and storing these large volumes of residue still remains an increasing environmental risk (Burke et al., 2013). Ecological reconstruction is a promising way forward for the remediation of bauxite residue on a large scale, reducing environmental risks (Wu et al., 2019; Xue et al., 2016). Nevertheless, bauxite residue has high salinity (electrical conductivity ≈ 7.4 mS cm−1) and alkalinity (pH ≈ 11.3, exchangeable sodium percentage ≈ 69%), and a fine particle composition (Gräfe, Power, & Klauber, 2011; Kong et al., 2017); these properties result in poor aggregate structure and water holding capacity, which limit plant survival (Rashti, Esfandbod, Phillips, & Chen, 2019; You, Zhang, Ye, & Huang, 2019).
During the last few decades, a number of studies have focused on the removal of alkalinity and salinity in the residues prior to field rehabilitation. It has been increasingly recognized that for successful cover establishment on mine residues, the process of aggregate formation and soil development is critical (Cavalcante, de Castro, Chaves, da Silva, & de Oliveira, 2019). Soil aggregates are the basic unit of soil structure and aggregate stability affects the exchange of water, nutrients, gases and heat in soil, as well as the growth and metabolism of animals and microorganisms (Papadopoulos, Bird, Whitmore, & Mooney, 2009; Tang, Mo, Zhang, & Zhang, 2011; Yang, Liu, & Zhang, 2019). Various methods including wet sieving and Le Bissonnais' (LB) method have been proposed to characterize aggregate formation due to the complexity of mechanisms on particle aggregation or disintegration (Almajmaie, Hardie, Acuna, & Birch, 2017; Barthes & Roose, 2002). Among them, LB's method can simulate different wetting conditions and energies to identify different disaggregation mechanisms. However, these tests of aggregate stability which involve disruption by shaking in water or ethyl alcohol for a standard period of time do not exhibit the rate of aggregate slaking and the disintegration behavior of the intermediate stages (Fristensky & Grismer, 2008). Field, Minasny, and Gaggin (2006) used a combined method of ultrasonication and sieving to destroy soil aggregates and found that aggregate disintegration may be modeled as a first-order reaction to represent aggregate behavior. Mason, Greene, and Joeckel (2011) observed that laser diffraction measurements could identify the roles of distinctly different processes involved in the disintegration of aggregates and their influence on rates of disintegration. On the basis of these observations, the researchers developed standard procedures whereby soil samples were circulated through a laser diffraction analyzer for a period of 180 min and analyze the changes of particle size distribution (PSD) at short time intervals (Kasmerchak, Mason, & Liang, 2019). However, to date, little research focused on evaluating aggregate behaviour of bauxite residue, which may be critical to understanding aggregate formation and its stability. Furthermore, whether laser diffraction method could be used to characterize and quantify contrasts in aggregate stability and the rate of aggregation disintegration in bauxite residue following different treatments was still unknown.
Dispersion or flocculation of aggregates was related to pH, electrolyte and exchangeable base concentrations (Bronick & Lal, 2005). Curtin, Steppuhn, Selles, and Mermut (1995) found that Na addition caused clay expansion and disintegration of unstable aggregates. Le Bissonnais (1996) observed that the cation hydration radius and valence states were important factors which affected aggregate stability; multivalent cations had a strong flocculation effect, while monovalent cations had a strong dispersion effect. Furthermore, the interaction force between soil particles was the intrinsic driving force for agglomerate fragmentation (Li et al., 2013). Compared to the various external forces in the erosion theory, the internal forces, including electrostatic repulsion between soil particles, were more able to determine soil disaggregation (Hu et al., 2015). According to the classical Derjaguin–Landau–Verwey–Overbeek (DLVO) theory of colloidal particle interaction, electrostatic repulsion was controlled by the electric field around the particle, and electric field intensity was determined by the ion interface reaction characteristics (Santos & Levin, 2011). Cations in bauxite residue pore water are dominated by Na+ and Ca2+ (Xu, Ding, Kuruppu, Zhou, & Biswas, 2018). However, limited understanding was available on surface electrochemical properties, which may affect aggregate behaviour of bauxite residue directly.
Calcium-contained solid wastes (e.g., gypsum, phosphogypsum) have been applied to ameliorate physical and chemical properties of bauxite residue and support plant growth. However, understanding the potential mechanisms of Ca2+/Na+ on aggregate behaviour and its stability for bauxite residue remain scarce. The objectives of this research were to (1) investigate aggregate size distribution and its stability using LB's method following Ca2+ or Na+ addition; (2) analyze specific surface area and surface charge properties of residue aggregates using the combined determination method; (3) quantify the disintegration behavior of residue aggregates using laser diffraction analysis and (4) develop its potential applications and guidance for rehabilitation on the degraded disposal areas.
2 MATERIALS AND METHODS
2.1 Materials
Fresh bauxite residue was collected to a depth of 20 cm from a bauxite residue disposal area in Central China. The climate is warm temperate continental monsoon, with an average daily temperature of 12.2–14.8°C and mean annual precipitation of 600–700 mm. Samples were subsequently stored in polyethylene bags, returned to the laboratory, air-dried at room temperature for 2 weeks and then passed through a 2 mm sieve prior to analysis.
2.2 Aggregate disintegration
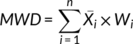 ((1))
((1))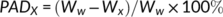 ((2))
((2))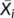 was the mean diameter over the adjacent sieves (mm), Wi was the percentage of residue aggregates in the size range and n was the number of sample sieves. Ww was the percentage of >X mm residue aggregates after wet sieving using deionized water, and Wx was the percentage of >X mm residue aggregates after wet sieving using the electrolyte solution.
was the mean diameter over the adjacent sieves (mm), Wi was the percentage of residue aggregates in the size range and n was the number of sample sieves. Ww was the percentage of >X mm residue aggregates after wet sieving using deionized water, and Wx was the percentage of >X mm residue aggregates after wet sieving using the electrolyte solution.2.3 Surface electrochemical properties of residue aggregates
The surface electrochemical properties of residue aggregates were determined using the combined determination method (Li et al., 2011). Bauxite residue, which was treated by different concentrations of NaCl or CaCl2 solutions, was separated through a 0.05 mm sieve and the <0.05 mm fractions were dried at 105°C for 24 h. The fractions were then added to a HCl solution (0.1 mol L−1 (v:w = 5:1), oscillated for 24 hours at 25°C, centrifuged at a speed of 4,000 rpm for 5 min. This process was repeated five times, and the samples were washed five times using deionized water to remove excess H+ in the suspension and separated to obtain the H+-saturated residue samples. Five grams of the H+-saturated samples were added into a 150 mL triangular bottle and mixed with an equal volume (10 mL) of Ca(OH)2 (0.01 mol L−1) and NaOH solution (0.015 mol L−1). The mixtures were oscillated for 24 hours and subsequently adjusted to pH 7 using HCl solution (1 mol L−1). In the equilibrium state, when the pH value of the suspension was higher than 7, it indicated that all of the previously absorbed H+ had been replaced by Ca2+ and Na+ (Yu, Zhang, Zhang, Xin, & Li, 2017). Once the equilibrium was reached, the suspension was then centrifuged and the concentrations of Ca2+ and Na+ in the supernatant were then determined using inductively coupled plasma – mass spectrometry (ICP-MS).
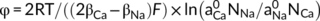 ((3))
((3))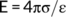 ((4))
((4))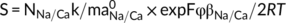 ((5))
((5))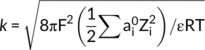 ((6))
((6))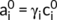 ((7))
((7))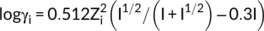 ((8))
((8)) ((9))
((9)) ((10))
((10))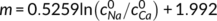 ((11))
((11))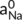 and
and 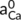 (mol L−1) respectively for the activity of Na+ and Ca2+ in the solution at equilibrium, NNa and NCa (mol) are the adsorption capacities of Na+ and Ca2+ in the residue, respectively.
(mol L−1) respectively for the activity of Na+ and Ca2+ in the solution at equilibrium, NNa and NCa (mol) are the adsorption capacities of Na+ and Ca2+ in the residue, respectively. 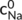 and
and 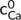 (mol L−1) were concentration of Na+ and Ca2+ in the solution at equilibrium, βNa and βCa were correction coefficients for correcting the effective charge amount of Na+ and Ca2+, respectively.
(mol L−1) were concentration of Na+ and Ca2+ in the solution at equilibrium, βNa and βCa were correction coefficients for correcting the effective charge amount of Na+ and Ca2+, respectively.2.4 Analysis of aggregate behaviour
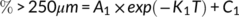 ((12))
((12))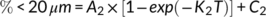 ((13))
((13))2.5 Morphological analysis
Micro-morphological studies of <0.05 mm aggregates from the three different treatments (untreated residue, residue treated by 50 mmol L−1 NaCl solution and residue treated by 50 mmol L−1 CaCl2 solution) were scanned using a scanning electron microscope, equipped with energy-dispersive X-ray spectroscopy (ESEM, Quanta-200). The samples were splutter coated with Au prior to scanning using a gaseous secondary electron detector (GSED) field emission gun.
2.6 Statistical methods
All of the analyses were performed in triplicate and the obtained data were statistically treated with Excel 2010 and IBM® SPSS® Statistics version.21. The three-dimensional surface map of the PSD of bauxite residue aggregates after different ion treatments was drawn by MATLAB R2017b. Analysis of variance (ANOVA) and homogeneity of variance tests were performed to determine statistical differences in aggregation properties and surface electrochemical properties under Ca2+ and Na+ addition. A post hoc least significant difference lest was performed when there was homogeneity, while Dunnett's T3 test was carried out in case of no homogeneity. Non-linear fitting model was used to match the trend of aggregates size distribution during the circulation period. All figures and lines representing first-order models were constructed by ORIGIN 9.0.
3 RESULTS
3.1 Aggregate size distribution and stability
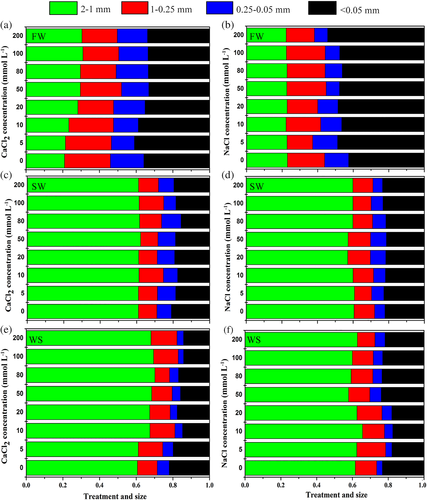
Aggregate size distribution of bauxite residue following different treatments is presented in Figure 1. For the FW test (simulating the slaking process), <0.05 mm aggregates were the major fraction. With CaCl2 treatment, the proportion of 0.25–2 mm aggregates ranged from 45.92 to 52.02%, which were higher than in untreated residues. With increasing Na+ concentration, the proportion of <0.05 mm microaggregates increased from 42.5 to 54.4%. For the SW test (simulating differential clay swelling processes) and the WS test (simulating the mechanism of breakdown processes), 1–2 mm aggregates were the major fractions. With CaCl2 treatment, the proportions of 1–2 mm aggregates increased from 60.9 to 62.2% and 69.8% for the SW and WS test, respectively.
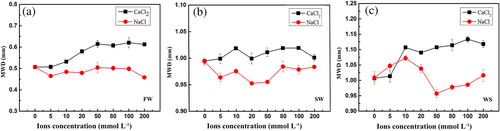
MWD is a characteristic indicator used to evaluate aggregate stability and a large MWD indicates improved aggregate stability (Cui et al., 2019; Mbagwu & Auerswald, 1999; You, Dalal, & Huang, 2018). Variation in MWD following NaCl and CaCl2 additions are presented in Figure 2. For the three different tests, CaCl2 addition increased MWD due to the accumulation of larger sized fractions. With increasing NaCl concentration, MWD decreased, although this did fluctuate. When the electrolyte concentration was 50 mmol L−1, MWD of CaCl2 treated samples reached a relatively high value, while a relatively low value occurred for NaCl treatments.
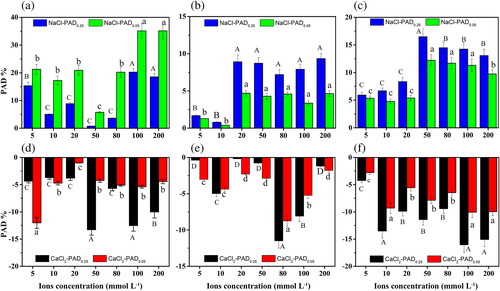
The percentage of aggregate destruction (PADx), which is the fraction of >x mm aggregates after wet sieving, may be used to evaluate variation in water-stable aggregates (Guber, Pachepsky, & Levkovsky, 2005). A positive value for PADx indicates dispersion, while a negative value indicates aggregation. The larger the absolute value of PADx, the stronger the corresponding effects. Variation in PADx in the treated residues is presented in Figure 3. The values of PAD0.25 and PAD0.05 of NaCl treated samples were positive, while negative values were observed in CaCl2 treated samples. Overall, with the increase of CaCl2 concentration, the absolute values of PAD0.25 were larger than that of PAD0.05 following FW, SW and WS treatment.
3.2 Aggregate surface properties
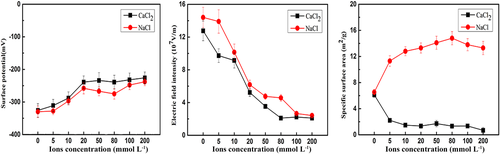
Variations in pH of bauxite residue microaggregates (<0.05 mm) following the different electrolyte treatments are presented in Table 1. pH declined gradually with less than 20 mmol L−1 Ca2+, but decreased sharply to approximately 8 when Ca2+ exceeded 50 mmol L−1. Effects of Ca2+/Na+ on surface properties of <0.05 mm residue aggregates are presented in Figure 4. With an increase in electrolyte concentration, both electric field intensity and surface potential decreased. The initial electric field strength of microaggregate particles was about 108 V m−1. With the increase of the electrolyte concentration, the electric field strength gradually decreased to about 107 V m−1. At the same electrolyte concentration, the electric field strength of the bauxite residue particles in the Ca2+ system was lower than that of the Na+ system. The effects of Ca2+ and Na+ on the specific surface area of bauxite residue micro-aggregate particles showed opposite results. With the increase of Ca2+ concentration, the specific surface area of microaggregate particles decreased from 6.12 m2 g−1 to 0.68 m2 g−1, and with the increase of Na+ concentration, the specific surface area increased to 13.3 m2 g−1.
| pH | Electrolyte concentration (mmol L−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 20 | 50 | 80 | 100 | 200 | |
| Na+ | 11.18 ± 0.21a | 10.89 ± 0.13bc | 10.94 ± 0.12b | 10.69 ± 0.22c | 10.60 ± 0.30e | 10.66 ± 0.20 cd | 10.64 ± 0.19f | 10.67 ± 0.22 cd |
| Ca2+ | 11.13 ± 0.31a | 10.82 ± 0.15b | 10.65 ± 0.40bc | 10.16 ± 0.21c | 8.21 ± 0.32d | 7.64 ± 0.13f | 7.72 ± 0.34e | 7.79 ± 0.10e |
- Note: Mean ± SD values (n = 3). Mean values in a row followed by different letters are significantly different at the 0.05 probability level.
3.3 Laser diffraction analysis of aggregate behavior
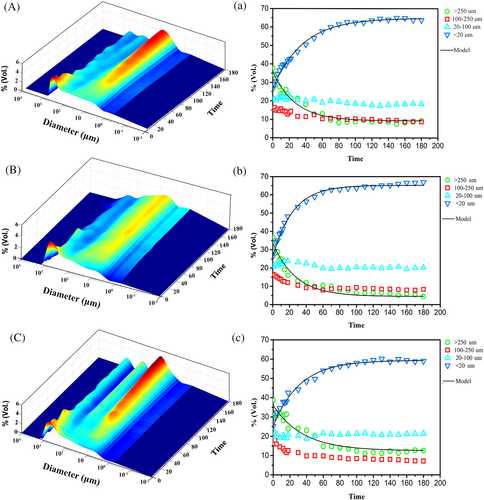
Aggregate behavior results are depicted as either continuous surfaces plots and scatter plot which portray changes in the overall size distribution over time (Figure 5a-c), or in the proportion of individual size fractions (Figure 5a-c). This represents a detailed view of aggregate disintegration and hydrodynamic behaviour in deionized water and other solution electrolytes. For the different treatments, the fastest disintegration rate in the first 20 min decreased as follows: Na+ > untreated>Ca2+ (Figure 5a-c). PSD of the three samples varied in the first 60 min and then approached a stable state. Variation in PSD in the salt solutions was different, which indicated that residue aggregates exhibited diverse disintegration and hydrodynamic behaviours under the effects of different ions. For the untreated samples, the initial flat peak of PSD occurred in the range of 2–250 μm after a 20-min circulation period (Figure 5a). For NaCl treated residues, the initial PSD peak disintegrated following a 30-min circulation, but then stabilized and persisted during the remaining circulation time (Figure 5b). During circulation, a peak of 10–50 μm appeared, revealing that fractions >250 μm disintegrated into 20–50 μm smaller particles. This indicated that large size aggregates may disintegrate into microaggregates in the presence of Na+. For CaCl2 treated residues, the initial peak of PSD collapsed at a circulation time of 40 min, and the peak height remained at approximately 1.5% at the end of the circulation period (Figure 5c). The first-order rate equation parameters for residue aggregates in different treatments are presented in Table 2. The group treated by Ca2+ had a lower A1 and K1 value, which indicated that disintegration rate of large aggregate was slower than that following Na+ addition. The lower C1 value from the group treated by Na+ indicated a faster disintegration rate during a short period of circulation. Furthermore, the group treated by Ca2+ had lower values of A2, K2 and C2, which indicated that the increase rate of the proportion of fine particles was slower. Meanwhile, the group treated by Ca2+ had a lower value of a/b than which by Na+, showing that Ca2+ could stimulate microaggregates formation. After circulation for 180 min, variations in C1 and C2 values revealed that Ca2+ addition increased the content of water-stable aggregate.
| Treatment | A1 | K1 | C1 | R12 | A2 | K2 | C2 | R22 | a | b | a/b |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Untreated | 26.61b | 0.037a | 9.21b | 0.97a | 38.27b | 0.027c | 26.55a | 0.99a | 25.64b | 37.22a | 0.69b |
| Na+ addition | 29.28a | 0.036a | 4.46c | 0.96a | 39.20a | 0.042a | 26.05b | 0.99a | 28.23a | 37.60a | 0.75a |
| Ca2+ addition | 23.50c | 0.028b | 12.51a | 0.94b | 34.17c | 0.031b | 25.22c | 0.98b | 22.85c | 33.12b | 0.69b |
- Note: Mean values in a column followed by different letters are significantly different at the 0.05 probability level. K1 and K2 are rate constants; A1 and A2 are rate coefficients; C1 is the final percentage of >250 μm fractions; C2 represents the initial percentage of <20 μm fractions in the residue aggregates; a = A1*exp(−k1); b = A2*exp(−k2); R12 and R22 are the fitness of the curves.
3.4 Residue micromorphology
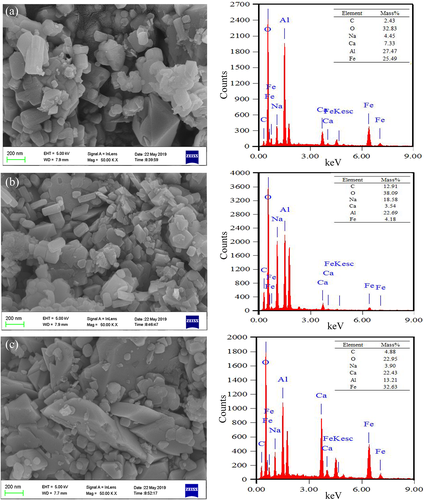
The presence of Ca or Na changed the surface properties, affected aggregate behavior and varied aggregate size distribution of bauxite residue. Micro-morphology and elemental distribution of <0.05 mm residue aggregates following three treatments are presented in Figure 6. Scanning electron microscope imaging of untreated samples revealed that 0.2–1 μm particles were the major fraction present. Control residue microaggregates had sheet or prismatic structures and there were relatively few fine fragments. With addition of NaCl, microaggregates sizes became smaller and the fine fragments increased. The major fractions of Ca-treated microaggregates were 0.5–1 μm particles and their structure was converted from a sheet-like to prismatic-like form. The variation in residue micro-morphology directly reflected the input of Ca2+, by revealing that it could improve aggregate structure and enhance aggregate stability of residues. Similarly, using a combination method including scanning electron microscope and synchrotron-based X-ray micro-computed tomography, gypsum has been shown to improve particle size of residue aggregates, enhancing the number of large pore spaces (Xue, Ye, et al., 2019). Multivalent ions such as Ca2+ may bind clay particles and organic matter to enhance particle agglomeration, or Ca2+ may exist as carbonate precipitates to form carbonate coatings and bind mineral particles together to inhibit clay dispersion (Jiang, Séquaris, Vereecken, & Klumpp, 2012). Kong et al. (2017) investigated acid transformation of bauxite residue, they found that gypsum addition promoted the leaching of sodium ions and accelerated the 0.2–1 μm particle fraction in 2–5 μm aggregates due to calcium's positive effect. According to energy dispersive spectrometer (EDS) analysis, Na, Ca, Al and Fe on the surfaces of residue aggregates were the major chemical elements. The mass fractions of Na, Ca, Al and Fe on the surfaces accounted for 4.45%, 7.33%, 27.47%, and 25.49%, respectively. Addition of CaCl2 significantly accumulated Ca while reducing Na. This indicated that Ca2+ could replace exchangeable Na+ on the surface of the particles and the extra Na+ would be leached out in solution. Ca2+ on the aggregate surface may then improve aggregate structure and micro-morphology of the residues.
4 DISCUSSION
4.1 Effect of Ca2+/Na+ on the formation and stability of residue aggregates
The improved aggregate structure is critical to soil formation process in bauxite residue. Ca2+ and Na+ were the major salt cations in bauxite residue (Xue, Wang, et al., 2019), while the difference of their contents directly affected the formation of stable aggregates (Bronick & Lal, 2005). Variations on MWD values indicated that Ca2+ addition enhanced aggregate stability of bauxite residue. Amezketa (1999) pointed out that Ca2+ could inhibit clay dispersion by replacing Na+ in clay and aggregates, thereby accumulating to aggregate stability. Some researches demonstrated that calcareous materials including gypsum and phosphogypsum were effective amendments to improve aggregate stability of bauxite residue (Santini et al., 2015). Variations on PAD values could reflect the effects of Ca2+ or Na+ on the contents of aggregates within different sizes. PAD values following CaCl2 treatment were positive, which indicated that the addition of Ca2+ accumulated the fractions of both >0.25 mm and >0.05 mm residue aggregates. Instead, Na+ in the residue solution contributed to repulsive charges and dispersive clay particles. Furthermore, the effect of Ca2+ on the proportion of 0.25–0.05 mm aggregates were more significant than that on >0.25 mm aggregates based on the absolute values of PAD0.25 and PAD0.05 following CaCl2 treatment as a whole. It indicated that CaCl2 addition was beneficial to the aggregation of clay-size particles and the formation of larger-size microaggregates. Tisdall and Oades (1982) proposed the hierarchical formation model of soil aggregates and found that Ca2+ could improve particles flocculation through cationic bridging with clay particles and organic carbon. Smith, Tongway, Tighe, and Reid (2015) observed that Ca2+ played a dominant role in microaggregate stabilization through its capacity to increase the availability of Ca2+. Zhu et al. (2017) observed that gypsum was beneficial to the flocculation of 0.05–0.02 mm residue microaggregate, which was consistent with the result obtained in this study.
The composition of ions significantly varied soil surface properties including electric field intensity, surface potential and specific surface area of aggregates, which may affect particles aggregation. Ca2+ addition decreased the pH of residue solution, while Na+ had little effects on the variation of pH values (Table 1). The major alkaline anions of bauxite residue included OH−, CO32− and Al(OH)4−. Ca2+ addition could combine with the alkaline anions to form indissoluble solids including calcite (CaCO3) and hydrocalumite (Ca4Al2(OH)12CO3), and then decrease the pH (Gräfe et al., 2011). Kong et al. (2017) observed that gypsum application significantly increased Ca2+ content and decreased the pH of the supernatants of the treated residues. Furthermore, Ca2+ could produce a stronger compression effect on the electric double layer than that of Na+ (Parsons, Boström, Nostro, & Ninham, 2011; Pashley, 1981), which resulted in a lower surface potential of residue microaggregates following Ca2+ addition.
The results obtained from laser diffraction analysis could effectively reveal aggregate formation in bauxite residue. Following the first 10 min of circulation, the 100–250 μm aggregate fraction fell sharply, but then slowly declined. This reflected that the 100–250 μm aggregates were rapidly disaggregated into <100 μm aggregates, followed by slow disintegration of the latter into primary mineral particles and smaller microaggregates. Furthermore, the 20–100 μm aggregates varied monotonically, representing a slightly decrease in microaggregates with small particle size following long-term circulation. This indicated that >100 μm fractions disaggregated into finer particles under the action of hydraulic power. In contrast, the proportions of <20 μm aggregates increased rapidly with almost all within 60 min, clearly reflecting disintegration of microaggregates. As the mechanical energy applied over time was difficult to quantify, Mason et al. (2011) used circulation time as a proxy for applied energy to model aggregate breakdown. The parameters estimated for the first-order model was related to disintegration behavior of residue aggregates following laser diffraction analysis. Integrated parameters were used here to evaluate the disintegration rate of large aggregates and increase rate of fine particle fractions. Furthermore, the value of a/b could also effectively reflect the details in disintegrating process of aggregates within other sizes to some extent, which may not be visually detected in continuous surface three-dimensional plots. The values of a/b following different treatment were less than 1, which indicated that the increase rate of <20 μm fractions was higher than the disintegrating rate of >250 μm fractions. Meanwhile, Ca2+ addition decreased the disintegration rate of >250 μm aggregates and the formation rate of <20 μm aggregates.
4.2 Quantification of pedogenic aggregate behaviour
The wet sieving method including Yoder's method and Le Bissonnais' method has been routinely used to evaluate aggregate stability and disaggregation mechanisms. Compared to Yoder's method, Le Bissonnais' method was more appropriate to determine the susceptibility to disaggregation mechanisms of residue aggregates including slaking (FW test), differential swelling of clay (SW test) or mechanical breakdown (WS test) (Zhu et al., 2016). In this study, aggregate size distribution from the modified LB's method showed that Ca2+ increased the proportion of water-stable aggregates (>0.25 mm) and improved aggregate stability, while Na+ resulted in disaggregation of larger sized aggregates (Figure 1). For the residues treated by CaCl2 solution, FW was the most efficient disaggregation mechanism, SW was intermediate while WS disintegrated stable aggregates the least based on the results presented in Figure 2. Therefore, residue aggregates were highly sensitive to slaking, less sensitive to differential clay swelling, and least sensitive to mechanical breakdown. Compared with the SW test, the MWD values increased more significantly for the FW and WS test with increasing Ca2+ concentration. It revealed that Ca2+ addition could enhance aggregate stability of bauxite residue effectively and protect the aggregates from disintegration by slaking or mechanical breakdown.
The electrochemical properties including pH, surface potential and electric field intensity were determined by the surface chemistry of residue minerals and influenced particles aggregation. Kong et al. (2018) observed that the fraction of fine particles decreased and macroaggregates formed, providing a shallower zeta potential curve and a lower surface area of bauxite residue undergoing natural rehabilitation. Hu et al. (2015) calculated the surface potentials of soil by the similar method and found that strong electric field in soil induced aggregates sudden breakdown, while weak electric field induced slow breakdown. With the decrease of particle surface potential, the aggregates were difficult to breakdown and release more small particles. In this study, compared with NaCl treatment, CaCl2 treated residue aggregates had lower surface potential and larger specific surface area, which indicated that CaCl2 treated residue aggregates were more stable. The variations on surface electrochemical properties of residue aggregates could reflect the changes of aggregate stability and its morphology, which indicated that the method was effective to analysis the potential mechanisms of the formation of stable aggregates in bauxite residue.
Tisdall and Oades (1982) proposed a hierarchical model according to the soil aggregate formation process; principally, primary particles (<20 μm) and the cements bond together to form microaggregates (20–250 μm), and thereby form larger aggregates (>250 μm). Plots of fine particles increased and continuous surface plots changed, which showed that aggregate disintegration rates and the proportions of water-stable aggregates were significantly different following Ca2+ or Na+ addition. The >250 μm aggregate fraction mainly included macro-aggregates, medium to coarse sand grains and larger coarse mineral fragments. This fraction dispersed rapidly, with at least half of the decline occurring in the first 20 min and almost all of it within approximately 60 min (Figure 5a-c). The 100–250 μm aggregate fraction included medium sand grains and large size micro-aggregates. In this study, residue aggregates with a large size mainly disaggregated into <20 μm fractions according to the trends of these fractions with circulation time. The obtained results demonstrated that laser diffraction method could determine the rate at which aggregates disintegrate and provide valuable information on pedogenic aggregate behavior of residues. Furthermore, first-order model provided a good fit to aggregate disintegration of bauxite residue during 180-min circulation. It indicated that the model could be applied to analysis disintegration behavior of residue aggregates as it provided straightforward indicators of the disintegration rate and the simple comparison between results for determining structural stability of residue aggregates.
4.3 Potential theoretical guidance for rehabilitation
Ecological reconstruction, not only revegetation, should be the beneficial strategy to reduce potential environmental risks. Many researchers have demonstrated that gypsum or phosphogypsum is effective amendments to alleviate the high alkalinity of bauxite residue (Cusack et al., 2018; Jones, Haynes, & Phillips, 2015). In both experimental or field research, 2–4% (w/w) gypsum or phosphogypsum were added to improve physic-chemical properties of bauxite residues (Bray et al., 2018). The additive amount of gypsum or phosphogypsum from these literatures usually took into account rehabilitation costs and alkaline removal effects. Little researches focus on the relationships between the addition amount of calcareous materials and aggregate stability, although the formation of stable aggregates in bauxite residue is essential to realize soil formation and ecological reconstruction.
Actually, about 50 mmol L−1 was the optimum addition concentration of Ca2+ into the residues due to the comprehensive effects on aggregate size distribution, aggregate surface properties and micro-morphology in this study. It was assumed that the liquid–solid ratio in the actual application process was 5:1, then the additive amount of CaCl2 should be 2.8%. Considering the significant differences in solubility of CaCl2 and CaSO4, the addition of CaSO4 should be larger than 2–4% (w/w) to stimulate aggregate formation in the residues. It was worth noting that the addition of excess gypsum may cause potential environmental risks and financial pressure. Organic wastes including poultry manure, vermicompost and biomass could stimulate the formation of water-stable aggregates, and should be effective candidates to enhance aggregate stability of bauxite residue. Zhu et al. (2017) found that the combined addition of gypsum and vermicompost had more significant effects on porosity, the proportion of water-stable aggregates and aggregate stability than gypsum addition. Therefore, the ameliorants should combine 2–4% gypsum and other organic amendments due to cost, and the rehabilitation effects on both chemical and physical properties.
5 CONCLUSIONS
Aggregate behaviour and stability of bauxite residue as affected by Ca2+/Na+ additions were evaluated in this study. With increasing Ca2+ content, MWD increased due to the flocculation of silt-sized microaggregates. Water-stable aggregates >0.25 mm disintegrated significantly following Na+ addition. Surface potential and electric field intensity of residue microaggregates gradually decreased with increasing electrolyte concentration. Furthermore, surface potential and electric field intensity in the Ca2+ system was lower than that in the Na+ system, which may contribute to aggregate flocculation. Laser diffraction analysis allowed for continuous monitoring of aggregate disintegration during 180 min of circulation in solutions. Aggregate size, structure and elemental distribution changed significantly following Ca2+/Na+ treatments. The findings will provide a combined approach to quantify the effects of typical electrolyte on the formation of stable aggregates, while improving the understanding of soil development in bauxite residue. Future research should focus on evaluating pedogenic aggregate behaviour of bauxite residue during the process of ecological rehabilitation at the field scale.
ACKNOWLEDGMENTS
This research received funding from the National Key Research and Development Program of China (No. 2019YFC1803604), the National Natural Science Foundation of China (No. 41701587), and the Fundamental Research Funds for the Central Universities of Central South University (No. 202045010).




