Radiofrequency Neurolysis of the Posterior Nasal Nerve: A Systematic Review and Meta-Analysis
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Abstract
Objective
Temperature-controlled radiofrequency neurolysis of the posterior nasal nerve (PNN) has been approved for use since 2020. This review synthesized the published data to assess its efficacy for treatment of chronic rhinitis.
Data Sources
Pubmed/Medline, Embase, Scopus, Web of Science.
Review Methods
A systematic search was conducted with no restrictions on publication years in April 2023. RCTs and prospective investigations that reported the reflective Total Nasal Symptom Score (rTNSS) outcome of radiofrequency neurolysis as a single procedure in chronic rhinitis patients were included. Pooled estimates for change in rTNSS from baseline at 3 months and responder rates (≥30% reduction in baseline rTNSS) at 3 and 6 months were obtained. Other outcomes, such as postnasal drip and cough scores, quality of life (QoL) measures, and adverse events were included for qualitative review.
Results
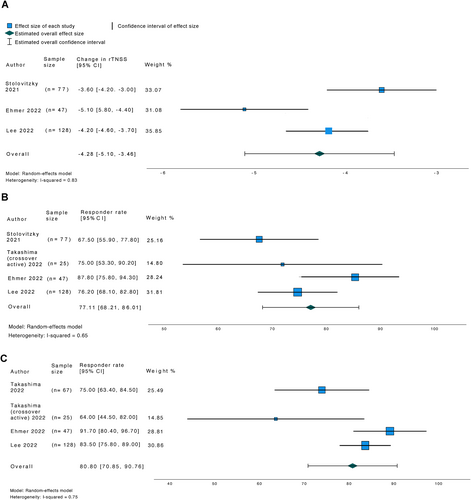
Five studies were included in the systematic review, of which four were included in the meta-analysis. A total of 284 participants underwent treatment. The pooled change in rTNSS score at 3 months was −4.28 (95% CI, −5.10 to −3.46). The pooled responder rate at 3 months was 77.11% (95% CI, 68.21%–86.01%) and at 6 months 80.80% (95% CI, 70.85%–90.76%). Postnasal drip and cough scores and QoL also improved significantly at follow up. A total of 36 adverse events were reported in 21 (7.4%) patients.
Conclusions
The findings from this review suggest that temperature-controlled radiofrequency neurolysis of the PNN is effective at treating chronic rhinitis symptoms and that it has an overall favorable safety profile. Laryngoscope, 134:507–516, 2024
INTRODUCTION
Chronic rhinitis is a common medical problem worldwide and can dramatically impair quality of life.1 Although medications are the mainstay treatment option, approximately 10%–22% of the patients with chronic rhinitis still have persistent symptoms despite medical therapy and may require further interventions.2 Historically, vidian neurectomy, which targets the vidian nerve, was offered for refractory rhinitis. Several systematic reviews have shown that vidian neurectomy is effective to reduce symptoms like rhinorrhea, but there are downsides to this treatment including the side effects of cheek and palate numbness and dry eyes.3, 4 Interventions that specifically target the post-ganglionic posterior nasal nerve (PNN) branches of the vidian nerve as they exit the sphenopalatine foramen help to reduce the morbidity associated with vidian neurectomy.5 These interventions range from surgical ablation of the PNN to minimally invasive options of cryotherapy, radiofrequency, or laser ablation of the nerve.
A previous systematic review by Balai et al, which evaluated the efficacy of these minimally invasive procedures, showed that these treatments improved chronic rhinitis symptoms.6 However, only one of their eight studies examined the effects of radiofrequency ablation of the PNN. Since the publication of this systematic review, neurolysis of the PNN with temperature-controlled radiofrequency has been gaining more interest. The Rhinaer system is a device designed for radiofrequency ablation of the PNN, targeting tissue in the region of the PNN near the posterior middle meatus and the superior portion of the posterior inferior turbinate. This technology monitors tissue temperature and automatically adjusts the radiofrequency current to maintain a therapeutic temperature of ~60°C without causing additional surrounding tissue damage. Since the approval of the Rhinaer system by the Food and Drug Administrations (FDA) in 2020, there have been several studies published that have investigated its efficacy in reducing chronic rhinitis symptoms.7-11 This systematic review seeks to evaluate the existing literature to determine if radiofrequency neurolysis of the PNN as a single procedure is effective in reducing symptoms among adult chronic rhinitis patients.
METHODS
Search Strategy and Study Selection
A comprehensive systematic search of four electronic databases (Pubmed/Medline, Embase, Scopus, and Web of Science) was performed in April 2023, with no restrictions on the years of publications. The search strategy was developed with the assistance of an Information Services Librarian. The full search terms for PUBMED/MEDLINE were ((“Radiofrequency Ablation”[Mesh] OR “radiofrequency ablation” OR neurolysis OR RhinAer OR (“posterior nasal nerve” AND ablation)) AND (“Rhinitis”[Mesh] OR Rhinitis OR “Posterior Nasal Nerve” OR Rhinitides OR “postnasal drip”)) NOT ((animals[mh] NOT humans[mh])); for EMBASE were (“rhinitis”/exp OR rhinitis OR “posterior nasal nerve” OR rhinitides OR “postnasal drip”) AND (“radiofrequency ablation”/exp OR “radiofrequency ablation” OR neurolysis OR rhinaer OR (“posterior nasal nerve” AND ablation)) NOT ((“animal”/exp OR “nonhuman”/exp) NOT “human”/exp); for Scopus and Web of Science were ((radiofrequency AND ablation) OR neurolysis OR RhinAer OR “posterior nasal nerve ablation” AND (rhinitis or rhinitides OR postnasal drip OR posterior nasal nerve)). We report the systematic review according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.
Following the initial search, all titles and abstracts were independently screened by two authors (A.Y. and B.T) for eligibility against the following inclusion and exclusion criteria. The PICOS inclusion criteria required that the article was a randomized controlled trial (RCT) or a prospective study that reported the reflective Total Nasal Symptom Score (rTNSS) outcome for chronic rhinitis patients treated with radiofrequency neurolysis of the PNN as a single procedure. All patients in the included studies were 18–85 years old with chronic rhinitis symptoms of at least 6 months and a total 24-h rTNSS score of ≥6, as well as ≥2 for moderate to severe rhinorrhea, and ≥1 for mild to severe congestion. Case series, review articles, articles written in non-English languages, and grey literature (e.g., conference abstracts) were excluded from the current investigation.
Data Extraction
Data extraction was performed independently by two authors (A.Y. and B.T.) with any discrepancies resolved by the senior author (K.H.) if present. Data on study design, inclusion and exclusion criteria, recruitment period, sample sizes, follow up times, patient demographic and clinical information, outcomes, outcome measurements tools, and adverse events were extracted. Only data from the treatment arm were included because three out of the five studies were single-arm prospective studies. Patient reported outcome assessments included rTNSS, postnasal drip and cough scores, nasal pain visual analog scale (VAS), mini Rhinoconjunctivitis Quality of Life Questionnaire (mini-RQLQ), and Quality of Life (QoL) survey. rTNSS is a validated instrument that measures four symptoms categories (nasal congestion, nasal itching, rhinorrhea, and sneezing), each on a 4-point scale (0 no symptoms to 3 severe symptoms) with a maximal total score of 12. All 5 studies used the change in rTNSS from baseline as either their primary or secondary endpoint. All five studies also reported a responder rate as one of their efficacy endpoints, defined as a minimal clinically important difference (MCID) of either ≥1 point rTNSS improvement from baseline or the more stringent criteria of ≥30% improvement in rTNSS relative to baseline.
Outcomes for Quantitative and Qualitative Synthesis
The primary outcome measure for the quantitative synthesis was the change in rTNSS score from baseline (pre-procedure) to 3 months of follow up. The secondary outcome measures were the responder rates defined as having a MCID of ≥30% improvement in rTNSS relative to baseline measured at 3 and 6 months.12 MCID of ≥1 point rTNSS improvement from baseline was not included in the quantitative synthesis because only two studies reported that outcome and at varied time points. Similarly, postnasal drip and cough scores, nasal pain VAS, QoL measures, and adverse events were only included in the qualitative synthesis because either the outcomes were not reported on a standardized scale or too few studies reported them if they were.
Data Analysis
We used random effects models to analyze the outcomes for the meta-analysis. To pool the responder rates at 6 months, the crossover active and the index active treatment groups from the Takashima 2022 study were included as separate studies because the results were reported separately. Heterogeneity was assessed through the I2 index which was defined as low (0%–50%), moderate (50%–75%), and high (75%–100%). Publication bias was not assessed given the few number of included studies. All statistical analyses were performed with SPSS software version 28 (IBM corporation, New York, USA) with 95% confidence intervals (CI) reported where applicable.
RESULTS
Study Characteristics
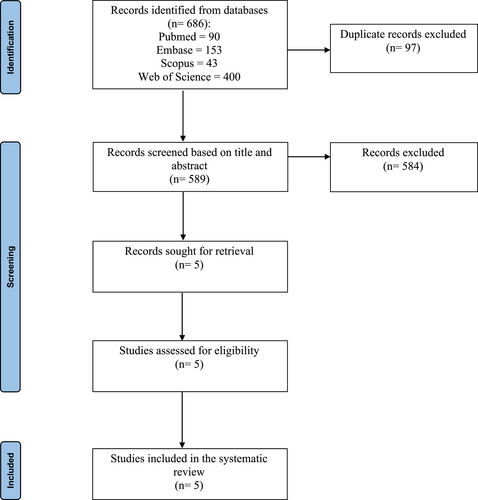
The initial database literature search yielded 686 articles. After screening based on title and abstract, five studies were retrieved for full text screening. All five studies were deemed eligible to be included in the systematic review for qualitative synthesis. The study selection process is detailed in Figure 1. There were two single-blinded RCT and three prospective single-arm uncontrolled and unblinded studies. The two RCTs reported results from the same study cohort at different time points; Takashima 2022 followed the index active treatment group of Stolovitzky 2021 until 12 months, and also incorporated a one-way crossover design where patients who were originally randomized to sham control could cross over to active treatment at the end of 3 months.8, 9 One of the prospective studies followed the same study cohort of another 12-month single arm study until 24 months.10, 11 All were multicenter institutional studies. Four out of the five studies were subsequently included in the meta-analysis for quantitative synthesis; the prospective extension study was excluded from the analysis because it did not report outcomes at 3 or 6 months.11
Characteristics of the Participants
Patients included in all five studies had chronic rhinitis symptoms of at least 6 months in duration, and a 24-h rTNSS score ≥6, as well as ≥2 for moderate to severe rhinorrhea, and ≥1 for mild to severe congestion. In addition, Ehmer 2022 and its prospective extension further required that patients were dissatisfied with medical management defined as an inadequate response (as judged by the patient) to at least 4 weeks usage of intranasal steroids before PNN neurolysis.10, 11 No data were provided on the mean duration or timing of the medical treatments prior to PNN neurolysis. Allergic, non-allergic, and mixed subtypes of rhinitis were all included, but seasonal allergies were excluded in three studies.7-9 Patients with altered anatomy of the posterior nose as a result of prior sinus or nasal surgery/injury or who had prior procedure/surgery for chronic rhinitis were excluded in three of the studies.7-9 All five studies were pragmatic in their design, which meant that concomitant medications for chronic rhinitis were not discontinued or controlled for.
The demographic and clinical characteristics of all included studies are summarized in Table I. The sample sizes of the studies ranged from 47 to 129 patients, with a pooled total of 284 patients who underwent treatment. The mean ages ranged between 57.3 and 57.9. The studies comprised of predominantly female (53.5%–63.6%) and white (89.6%–94.0%) participants. Of the three studies that reported the distribution of the subtypes of rhinitis, two reported allergic rhinitis to be the most common type (38.0%–42.0%) whereas the other study reported nonallergic rhinitis (72.1%). Baseline medication uses were reported by the three studies that did not require all patients to have inadequate responses to medical treatment prior to radiofrequency ablation; antihistamines (50.0%–72.7%) and intranasal steroid sprays (40.3%–64.1%) were the most frequently used medications, but it is not clear if all patients underwent some form of medical treatment prior to radiofrequency neurolysis.
| Stolovitzky 2021 | Takashima 2022 | Ehmer 2022 | Ehmer 2022 (extension) | Lee 2022 | |
|---|---|---|---|---|---|
| Study design | RCT | RCT | Single arm, prospective | Single arm, prospective | Single arm, prospective |
| Recruitment period | July 2020–December 2020 | July 2020–December 2020 | October 2018–June 2019 | October 2018–May 2021 | October 2020–May 2021 |
| Pre-treatment size, n | 78 | Index active: 77, crossover active: 27 | 50 | 47 | 129 |
| Post-treatment size, n | 77 | Index active: 67 (12 months), crossover active: 25 (6 months) | 47 | 34 | 128 (3 months), 123 (6 months) |
| Follow up period, months | 0–3 | Index active: 3–12, crossover active: 0–6 | 0–12 | 12–24 | 0–6 |
| Age, years (SD) | 57.3 (14.8) | Index active: 57.3 (14.8), crossover active: 57.4 (14.6) |
57.9 (11.9) | 57.9 (11.9) | 57.9 (13.4) |
| Percent female, n (%) | 49 (63.6) | Index active: 49 (63.6), crossover active: 16 (59.3) | 29 (58.0) | 21 (62.0) | 69 (53.5) |
| Race, n (%) | |||||
| White | 69 (89.6) | Index active: 69 (89.6), crossover active: 26 (96.3) | 47 (94.0) | 21 (94.0) | 117 (90.7) |
| Black | 5 (6.5) | Index active: 5 (6.5), crossover active: 0 (0) | 0 (0) | 0 (0) | 5 (3.9) |
| Asian | 1 (1.3) | Index active: 1 (1.3), crossover active: 1 (3.7) | 2 (4.0) | 1 (3.0) | 4 (3.1) |
| Other | 2 (2.6) | Index active: 2 (2.6), crossover active: 0 (0) | 1 (2.0) | 1 (3.0) | 3 (2.3) |
| Subtypes of rhinitis, n (%) | |||||
| Allergic | – | – | 21 (42.0) | 12 (38.0) | 10 (7.8) |
| Nonallergic rhinitis | – | – | 17 (34.0) | 10 (29.0) | 93 (72.1) |
| Mixed rhinitis | – | – | – | – | 1 (0.8) |
| Other | – | – | 12 (24.0) | 11 (32.0) | 25 (19.4) |
| Prior nasal surgery, n (%) | 27 (35.1) | – | – | – | 44 (34.1) |
| Baseline medication use, n (%) | |||||
| Antihistamines | 56 (72.7) | Index active: 46 (59.7), crossover active: 16 (59.3) | – | – | 64 (50.0) |
| Decongestants | 22 (28.6) | Index active: 12 (15.6), crossover active: 4 (14.8) | – | – | 32 (25.0) |
| Oral leukotriene inhibitors | 4 (5.2) | Index active: 5 (6.5), crossover active: 3 (11.1) | – | – | 14 (10.9) |
| Intranasal steroid spray | 34 (44.2) | Index active: 31 (40.3), crossover active: 15 (55.6) | – | – | 82 (64.1) |
| Intranasal anticholinergic spray | 19 (24.7) | Index active: 20 (26.0), crossover active: 5 (18.5) | – | – | 33 (25.8) |
- Abbreviation: SD, standard deviation.
Pooled Change in rTNSS Score and Responder Rates
Three studies reported the mean change in rTNSS score from baseline to 3 months (Table II). The pooled change in rTNSS score at 3 months was found to be −4.28 (95% CI, −5.10 to −3.46) (Figure 2). Four studies reported the responder rates at 3 and 6 months (Table II). The pooled responder rate at 3 months was 77.11% (95% CI, 68.21%–86.01%) and at 6 months 80.80% (95% CI, 70.85%–90.76%) (Figure 2). Heterogeneity was considered high for the pooled change in rTNSS score at 3 months (I2 0.83) and responder rates at 6 months (I2 0.75), and moderate for the pooled responder rates at 3 months (I2 0.65).
| Stolovitzky 2021 | Takashima 2022 | Ehmer 2022 | Ehmer 2022 (extension) | Lee 2022 | |
|---|---|---|---|---|---|
| Follow up time points | |||||
| 1 month | Yes | ||||
| 3 months | Yes | Crossover active: Yes | Yes | Yes | |
| 6 months | Index active: Yes, crossover active: Yes |
Yes | Yes | ||
| 12 months | Index active: Yes | Yes | |||
| 24 months | Yes | ||||
| rTNSS scores | |||||
| Baseline | 8.3 (95% CI, 7.9 to 8.7) | Crossover active: 8.0 (95% CI, 7.2 to 8.8) | 8.5 (95% CI, 8.0 to 9.0) | 8.4 (95% CI, 7.7 to 9.0) | 7.8 (95% CI, 7.5 to 8.1) |
| 3 months | Index active: 4.7 (95% CI not reported) Crossover active: 5.5 (95% CI not reported) |
3.4 (95% CI, 2.8 to 4.1) | 3.0 (95% CI not reported) | 3.6 (95% CI, 3.2 to 4.0) | |
| 6 months | Index active: 3.9 (95% CI not reported) Crossover active: 4.4 (95% CI not reported) |
3.3 (95% CI not reported) | 3.2 (95% CI not reported) | 2.9 (95% CI, 2.5 to 3.3) | |
| 12 months | Index active 3.5 (95% CI not reported) | 3.6 (95% CI, 3.0 to 4.3 | 3.4 (95% CI not reported) | ||
| 24 months | 2.9 (95% CI, 2.1 to 3.6) | ||||
| Change in rTNSS from baseline | |||||
| 3 months | −3.6 (95% Cl, −4.2 to −3.0) | Index active: −3.6 (95% CI, −4.2 to −3.0) | −5.1 (95% CI, −5.8 to −4.4) | −4.2 (95% CI, −4.6 to −3.7) | |
| 6 months | Index active: −4.4 (95% CI, −5.0 to −3.8) | −4.9 (95% CI, −5.5 to −4.3) | |||
| 12 months | Index active: −4.8 (95% CI, −5.5 to −4.1) | ||||
| 24 months | − 5.5 (95% CI, − 6.4 to – 4.6) | ||||
| Responder rate: rTNSS exceeding 1 point from baseline | |||||
| 3 months | 93.9% (95% CI, 83.5% to 97.9%) | ||||
| 6 months | 95.8% (95% CI, 86.0% to 98.8%) | ||||
| 12 months | 100% (95% CI, 92.4% to 100%) | ||||
| 24 months | 97.1% (95% CI, 85.1% to 99.5%) | ||||
| Responder rate: ≥30% improvement in baseline rTNSS | |||||
| 3 months | 67.5% (95% CI, 55.9% to 77.8%) | Crossover active: 75.0% (95% CI, 53.3% to 90.2%) | 87.8% (95% CI, 75.8% to 94.3%) | 76.2% (95% CI, 68.1% to 82.8%) | |
| 6 months | Index active: 75.0% (95% CI, 63.4% to 84.5%) Crossover active: 64.0% (95% CI, 42.5% to 82.0%) |
91.7% (95% CI, 80.4% to 96.7%) | 83.5% (95% CI, 75.8% to 89.0%) | ||
| 12 months | Index active: 80.6% (95% CI, 69.1% to 89.2%) | 80.9% (95% CI, 67.5 to 89.6%) | |||
| 24 months | 88.2% (95% CI, 73.4% to 95.3%) | ||||
| Postnasal drip score | |||||
| Baseline | Index active: 2.6 (95% CI 2.5 to 2.8) | 4.1 (95% CI, 3.6 to 4.6) | |||
| 3 months | |||||
| 24 months | 2.1 (95% CI, 1.7 to 2.5) | ||||
| Cough score | |||||
| Baseline | Index active: 1.6 (95% CI, 1.3 to 1.8) | 3.2 (95% CI, 2.7 to 3.6) | |||
| 3 months | |||||
| 24 months | 0.9 (95% CI, 0.5 to 1.3) | ||||
| Nasal pain VAS score | |||||
| Immediate post procedure | 2.1 (95% CI, 1.6 to 2.6) | Crossover active: 2.4 (95% CI, 1.4 to 3.3) | 18.1 (95% CI, 12.3 to 23.9) | 19.0 (95% CI, 14.7 to 23.2) | |
| 1 month | 0.8 (95% CI, 0.4 to 1.2) | Crossover active: 0.2 (95% CI, 0.0 to 0.3) | 5.0 (95% CI, 2.1 to 8.0) | ||
| 3 months | 0.6 (95% CI, 0.2 to 0.9) | Crossover active: 0.3 (95% CI, −0.1 to 0.6) | 4.4 (95% CI, 2.1 to 6.7) | ||
- Abbreviations: CI, confidence interval; rTNSS, reflective Total Nasal Symptoms Score; VAS, visual analog scale.
rTNSS Subscores, Postnasal Drip and Cough Scores
All five studies reported that each of the rTNSS subscore categories (nasal congestion, nasal itching, rhinorrhea, and sneezing) improved significantly from baseline to every follow up time point with treatment. In the RCT by Stolovitzky 2021, the decrease in rhinorrhea and congestion subscores at 3 months was significantly greater in the active treatment arm compared to the sham-control arm, whereas the decrease in the nasal itching subscore did not reach statistical significance.8 Postnasal drip and cough scores were measured in four out of the five studies, where these symptoms also improved significantly from baseline to each follow up time point (Table II).7, 9-11
Quality of Life (QoL) Measures
Two studies evaluated patient QoL outcomes, and both showed an overall improvement in QoL with treatment.7, 11 Lee 2022 used the mini-RQLQ, which is a validated questionnaire that consists of 14 questions in five domains (activity limitations, practical problems, nose symptoms, eye symptoms, and other symptoms), with a maximum score of 14 points. Patient QoL was found to be significantly improved at 3 months post-procedure with the mean change in mini-RQLQ score −1.6 (95% CI, −1.8 to −1.4, p < 0.001), a 53.3% improvement from baseline. At 6 months, the adjusted mean change in mini-RQLQ score was −1.8 (95% CI, −2.1 to −1.5), a 60.0% improvement. The prospective extension of Ehmer 2022 used a QoL survey with six questions regarding sleep, frustration/embarrassment, and overall well-being, and found that a significantly larger percentage of patients responded positively to the series of QoL questions after treatment, indicating an increase in QoL at 24 months.
Long Term Outcomes
The prospective extension of Ehmer 2022 was the only study that examined outcomes at >12 months (Table II).11 This study followed the original study cohort from 12 to 24 months. rTNSS score at 24 months was significantly improved from baseline with the mean change −5.5 (95% CI, −6.4 to −4.6, p < 0.001). The responder rate (≥30% reduction in baseline rTNSS) at 24 months was reported to be 88.2% (95% CI, 73.4%–95.3%).
Nasal Pain
Four studies reported nasal pain as measured by the VAS immediately post-procedure, and during follow up at 1 month and/or 3 months (Table II).7-10 Nasal pain was recorded on either a 10-cmVAS8, 9 or 100-point VAS7, 10 with 0 being no pain and 10 cm or 100 points indicating the worst pain possible. The post-procedure VAS reported in the studies were all lower than 20 points if on 100-point scale or lower than 2.5 cm if on the 10-cm scale. In the RCT by Stolovitzky 2021, nasal pain was not found to be significantly different between the active treatment group and the sham control at any time point.8 In all four studies, compared with immediately post-procedure, the VAS score was lower at either 110 or 3 months7 or both.8, 9
Adverse Events
A total of 36 adverse events were reported in 21 (7.4%) patients in the included studies. There were no serious adverse events related to the procedure or the device. The most common adverse events were nasal soreness and pain (five cases) and nasal mucosa changes (five cases), followed by sinusitis (four cases), nasal bleeding (three cases), and eye dryness (three cases). The full list of adverse events is listed in Table III.
| Stolovitzky 2021 | Takashima 2022 | Ehmer 2022 | Ehmer 2022 (extension) | Lee 2022 | Total | |
|---|---|---|---|---|---|---|
| Number of patients | Combined index active and crossover active: 5/105 = 4.8% | 8/50 = 16.0% | 0 | 8/129 = 6.2% | 21/284 = 7.4% | |
| Number of adverse events | 9 | Index active: 0 Crossover active: 1 |
16 | 0 | 10 | 36 |
| Type of adverse event | ||||||
| Ocular | ||||||
| Eye dryness | 1 | 1 | 1 | 3 | ||
| Eye pressure | 1 | 1 | ||||
| Nasal | ||||||
| Nasal soreness/pain | 2 | 1 | 2 | 5 | ||
| Nasal inflammation/redness | 1 | 1 | ||||
| Nasal mucosa changes | 2 | 2 | 1 | 5 | ||
| Postnasal drip | 2 | 2 | ||||
| Sneezing | 1 | 1 | ||||
| Nasal drainage | 1 | 1 | ||||
| Nasal bleeding | 1 | 1 | 1 | 3 | ||
| Nasal septum irritation | 1 | 1 | ||||
| Teeth pain | 1 | 1 | ||||
| Sinusitis | 1 | 1 | 2 | 4 | ||
| Nasal adhesion | 1 | 1 | ||||
| Oral | ||||||
| Mouth roof irritation | 1 | 1 | ||||
| Oropharyngeal pain | 2 | 2 | ||||
| Miscellaneous | ||||||
| Ear discomfort | 1 | 1 | 2 | |||
| Vasovagal reaction | 1 | 1 | ||||
| Headache | 1 | 1 | ||||
Risk of Bias
The two RCTs were deemed to be at an overall low risk of bias. The initial Ehmer 2022 was judged as low risk of bias. The extension of Ehmer 2022 was deemed as moderate risk of bias due to a lost to follow up rate of ≥20%. Lee 2022 was also considered to have a moderate risk of bias because of the shorter length of follow up at 6 months.
DISCUSSION
In the treatment of chronic rhinitis, minimally invasive interventions that specifically target the PNN have been gaining increasing attention due to their reduced morbidity compared to the traditional vidian neurectomy. A previous systematic review published in 2022 synthesized the evidence of these minimally invasive techniques and showed that these procedures led to an overall improvement in symptoms, but only one of their included studies examined the efficacy of radiofrequency ablation of the PNN. Since the introduction of Rhinaer in 2020, there have been multiple studies published regarding its treatment efficacy. The present systematic review and meta-analysis aimed to synthesize the data from these multiple studies to determine the efficacy of radiofrequency neurolysis alone. We have identified some evidence to suggest that temperature-controlled radiofrequency neurolysis of the PNN can lead to a clinically apparent improvement in chronic rhinitis symptoms, and that it is associated with an overall good safety profile.
The present meta-analysis of three studies found that the mean rTNSS score at 3 months improved by approximately four points compared with baseline. The RCT by Stolovitzky 2021 further demonstrated that the improvement in rTNSS was significantly greater in the active treatment arm than sham control at 3 months. Additionally, our meta-analysis of four studies demonstrated that approximately 77% of the participants had ≥30% reduction in baseline rTNSS score at 3 months and 81% at 6 months, indicating a clinically significant improvement in rTNSS at each time point. All studies reported a significant improvement for each rTNSS subscore (rhinorrhea, congestion, itching, and sneezing). The efficacy of radiofrequency ablation of PNN is thought to result from the interruption of efferent parasympathetic stimulation of the nasal mucosa, which leads to reduction in submucosal gland secretions and blood flow. Two of the five studies compared outcomes between allergic and nonallergic rhinitis, and found that an improvement in rTNSS from baseline was observed at all time points for patients of both subtypes.10, 11 It is possible that radiofrequency neurolysis also reduces neurogenic inflammation in patient with allergic rhinitis.
Besides radiofrequency neurolysis of the PNN, cryoablation of the PNN is another minimally invasive intervention for chronic rhinitis that has been gaining renewed interest. A qualitative systematic review by Kompelli et al revealed that the studies published between 1977 and 1997 all demonstrated subjective improvement in obstructive and rhinorrhea symptoms following treatment with cryoablation.13 More recent investigations including prospective single-arm and RCT studies for Clarifix cryotherapy published after 2017 have reported the mean change in rTNSS at 3 months to range between −3.10 and −4.00.14-18 Chang et al reported that the responder rate (similarly defined as ≥30% reduction in baseline rTNSS score) following cryoablation was 74.0% at 3 months and 78.9% at 6 months.16 The current review suggests that radiofrequency neurolysis of PNN has comparable efficacy as cryoablation in reducing chronic rhinitis symptoms, but this interpretation is limited by the few studies included in our meta-analysis. The improvement in rTNSS subscores reported separately by the included studies is consistent with that observed in studies using cryoablation of PNN.16 Additionally, the benefit seen in both allergic and nonallergic rhinitis groups with no significant difference between the two subgroups has been reported by several studies for cryoablation.16, 19, 20 An indirect comparison study between 2 RCTs for radiofrequency neurolysis and cryoablation also reported that the two methods were equally efficacious in their improvement in rTNSS at 3 months.21 Currently, there have not been any observational or RCT studies that directly compare cryotherapy and radiofrequency neurolysis of PNN in chronic rhinitis.
Radiofrequency neurolysis of the PNN is well tolerated with low nasal pain VAS scores post-procedure and during follow up. The RCT by Stolovitzky 2021 further demonstrated that nasal pain was not significantly different between the active treatment group and the sham control group at any time point. Only one study reported nasal pain VAS for cryoablation of the PNN, and in contrast, they found a higher post-procedure VAS at 75.9 ± 21.4 and a 3 months VAS at 36.0 ± 29.2.18
The overall safety profile for radiofrequency neurolysis is also favorable. Across the five studies included in our review, a total of 36 adverse events were reported in 21 (7.4%) patients. The most common adverse events were nasal soreness and pain and nasal mucosa changes. In comparison with cryoablation, Kompelli et al reported a similar complication rate (8.6%) but higher occurrences of nasal obstruction, crusting, and ear blockage.13 Contemporary studies for Clarifix cryotherapy reported that between 47.0% and 80.0% of the patients experienced side effects.15, 18 Headaches have been described as one of the most common adverse events after cryoablation.15, 16, 18 However, this was found to a lesser extent for radiofrequency neurolysis in our review. An advantage of using temperature-controlled radiofrequency neurolysis where a therapeutic temperature of ~60°C is maintained throughout the treatment with feedback monitoring to adjust the RF current accordingly is that it can limit the damage to the overlying mucosa of the treatment area and the surrounding tissue. In comparison with interventions that target the vidian nerve, a systematic review for vidian neurectomy including endoscopic and nonendoscopic approaches reported the overall rates of dry eyes to be 24.0% and maxillary cranial nerve paresthesia to be 8.2%.4 For endoscopic vidian neurectomy alone, another systematic review reported that the overall rate of dry eyes was 48.0% and of cheek/palate numbness was 6.3%.3 In our review, eye dryness was present in only three cases. There was no report of cheek/palate numbness, but teeth pain was reported in 1 case. Although there have not been any head-to-head observational or RCT studies that compared the safety and efficacy of radiofrequency neurolysis of PNN with other surgical interventions, the overall safety profile with the lack of potential serious side effects is promising.
Despite these encouraging results, these studies do have several weaknesses. The high heterogeneity of the included studies could be related to the fewer number of studies included. In addition, the meta-analysis of single-arm studies has inherent limitations related to the lack of a control group, which can introduce biases. Medication use was not controlled for by any of the studies due to the pragmatic nature of the studies to mimic realistic practice. Across all five studies, 5.4%–20.8% of the participants had an increased use in at least one medication class throughout treatment. The data were imputed to account for the potential confounding effect of medication increase on the outcome with no subsequent changes in the study conclusions. The studies were all comprised of racially homogenous participants with over 89% white, thus the conclusions may not be generalizable to the US population. Two of the five studies reported a follow up period of only 6 months or less, and one study that reported long-term 24 months outcomes had a high lost to follow up rate. Finally, the authors of all the studies were consultants for or received funding for the research from the medical device company.
Radiofrequency neurolysis of the PNN is effective in reducing chronic rhinitis symptoms. With its tolerability and favorable side effect profile, there appears to be a role for radiofrequency neurolysis in treating chronic rhinitis symptoms. Further studies are needed to characterize the long-term efficacy with additional RCTs that include a larger, more diverse patient population.
ACKNOWLEDGMENTS
The authors would like to acknowledge Dr. Melissa Lee Wilson MPH PhD for providing statistical support; and Karin Saric, Information Services Librarian, for assistance in developing the search strategy.
FUNDING INFORMATION
This study was supported by an educational grant from the Los Angeles County Medical Center Committee for Interns and Residents (CIR).




