The Rate of Occult Lesion Diagnosis in a Large Bell's Palsy Cohort
2023 Triological Society Combined Sections Meeting, Coronado, CA; January 26–28, 2023.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Abstract
Objectives
We characterize occult lesion diagnosis rates after initial Bell's palsy diagnoses.
Methods
A de-identified database of all facial palsy patients who presented to an extensive health care system across 22 years was created using Epic SlicerDicer. Among patients with Bell's palsy diagnoses, we extracted demographic and any subsequent occult lesion diagnosis data across various clinical sites. Descriptive and multivariable regression analyses comparing patients with occult lesion diagnoses made at different time points were included.
Results
Among the total 3912 facial palsy patients, 2240 had Bell's palsy diagnoses, of which 217 (9.7%) had subsequent lesion diagnoses at a median (IQR) of 12.3 (4.2, 23.8) months, consisting of cranial nerve neoplasms (62.2%), parotid gland neoplasms (34.1%), and cholesteatomas (3.7%). Although a large proportion of total lesions were diagnosed within the first 3 months (19.8%), 69.5% were diagnosed after 6 months. There were no demographic differences among patients diagnosed with different lesion types, but Asian patients were more likely to be diagnosed with occult lesions after 12 months after Bell's palsy diagnosis compared with white patients (odds ratio = 6.2, p = 0.001).
Conclusions
In one of the largest Bell's palsy cohorts to date, we identified a 9.7% occult lesion diagnosis rate at a median of 12.3 months after Bell's palsy diagnosis. These data underscore the importance of timely workup for occult lesions in cases of facial palsy with no signs of recovery after 3–4 months.
Level of Evidence
4 Laryngoscope, 134:911–918, 2024
INTRODUCTION
Bell's palsy is a debilitating disorder of facial nerve weakness diagnosed in approximately 40,000 individuals in the United States annually.1 Bell's palsy typically presents as rapid-onset unilateral facial palsy that recovers over several weeks to months. A diagnosis of exclusion, Bell's palsy, is believed to be caused by idiopathic reactivation of a latent herpes simplex virus within the geniculate ganglion of the facial nerve.2
The differential diagnosis of facial palsy is broad, which can make accurate diagnosis on initial assessment difficult. Facial palsy is most commonly caused by Bell's palsy, followed by trauma, infection, neoplasms, neurologic diseases, or stroke.3-5 Notably, occult lesions, especially parotid gland neoplasms, cranial nerve schwannomas, and cholesteatomas, can cause peripheral facial palsy like Bell's palsy by impinging on cranial nerve VII. In the absence of visible or palpable masses, otologic or neurologic deficits, or malignancy history, Bell's palsy is the most likely diagnosis, and patients are often treated with oral corticosteroids and antiviral medications and reassured that spontaneous recovery is likely.6
Patients who fail to recover or who have a fluctuating course of facial palsy may require multiple referrals before an occult lesion diagnosis is revealed. Although the importance of distinguishing Bell's palsy from occult lesions across different clinical sites has been reviewed in the literature, the actual rate of misdiagnosis of lesions in Bell's palsy is not well-established, and existing studies are limited by small cohorts.7-9 In the emergency department, reported misdiagnosis rates vary from less than 1%–20%.7, 8 In contrast, at an otolaryngology clinic, approximately one-third of a cohort of 32 patients with occult facial nerve schwannomas were initially misdiagnosed.10 Previously characterized risk factors for lesion misdiagnosis are atypical presentation, including non-palpable masses or no lymphadenopathy, and misinterpreted or false negative imaging and/or physical exam findings.7 As delays in lesion diagnosis can affect prognosis, a better understanding of the incidence and drivers of occult lesion misdiagnosis as Bell's palsy is needed.11, 12 In this retrospective study, we examined a cohort of 2240 patients diagnosed with Bell's palsy across different clinical sites over a 22-year period to characterize the incidence and nature of lesions detected after an initial Bell's palsy diagnosis.
MATERIALS AND METHODS
Patients diagnosed with International Classification of Disease (ICD), 10th Revision codes relating to facial weakness (G51.x, S04.5x, R29.810, and Q67.0), were identified from the University of California, San Diego, electronic medical records system between 1/1/2000 and 1/1/2022 via Epic SlicerDicer. We henceforth use the term “facial palsy” to refer to the spectrum of incomplete to complete facial weakness. The University of California San Diego Institutional Review Board deemed this study exempt from review and the informed consent requirement.
Patients with the G51.0 (Bell's palsy) ICD-10 code were identified. Age, sex, race, specialty site where the Bell's palsy diagnosis was made (including primary care, emergency department, otolaryngology, neurology, plastic surgery, ophthalmology, or another, unspecified clinical setting), and prescription antiviral and corticosteroid medication data were collected.
Any diagnoses of lesions that could affect facial nerve function were identified in the period after the initial Bell's palsy diagnosis. Specifically, the diagnoses of parotid gland neoplasms, cranial nerve neoplasms, and cholesteatomas were identified with ICD-10 codes D11.0, C07, D37.030, D36.11, D33.3xx, H93.3, C72.4x, and H71.xx. Lesion diagnoses that were made before the initial Bell's palsy diagnosis were excluded. In the data obtained via Epic SlicerDicer, we could not differentiate between facial and vestibular schwannoma diagnoses, so we use the umbrella term “cranial nerve neoplasms.” Time in months between Bell's palsy and occult lesion diagnoses was calculated, and patients were grouped based lesion diagnosis time in relationship to the initial Bell's palsy diagnosis: 0–12 months after the initial Bell's palsy diagnosis and over 12 months after Bell's palsy diagnosis. The 0–12-month timeframe was further subdivided into the following time groups with non-inclusive lower bounds: 0–3, 3–6, and 6–12 months.
Statistical Analysis
Patients’ demographic, clinical, and treatment characteristics were stratified by timeframe of lesion diagnosis after the initial Bell's palsy diagnosis and compared using one-way analysis of variance, Wilcoxon rank sum, and chi-squared tests. These data were also stratified by lesion type and compared using the aforementioned tests. Multivariable logistic regression models adjusting for demographic and clinical characteristics were created to determine factors associated with lesion detection status and presented as odds ratios (OR; 95% confidence intervals). All statistical analyses were performed with R version 4.1.3.
RESULTS
A total of 3912 facial palsy patients were identified over a 22-year period, of which 2240 (57.3%) had Bell's palsy diagnoses. The median (IQR) age among Bell's palsy patients was 58 (44, 69), 53% of patients were female, and 50% were white (Table I). A total of 217 (9.7%) patients were diagnosed with an occult lesion after an initial Bell's palsy diagnosis at a median of 12.3 (4.2, 23.8) months. The majority of lesions were cranial nerve neoplasms (62.2%), followed by malignant (30%) and benign (4.1%) parotid gland tumors, and cholesteatomas (3.7%).
| Characteristic | All Bell's palsy patients |
|---|---|
| N | 2420 |
| Age* | 58 [44, 69] |
| Gender† | |
| Female | 1273 (53%) |
| Male | 1147 (47%) |
| Race† | |
| White | 1198 (50%) |
| Asian | 230 (9.5%) |
| Black | 113 (4.7%) |
| Other or mixed | 743 (30.2%) |
| Bell's palsy diagnosis site† | |
| Emergency | 692 (20.6%) |
| Primary care | 444 (13.2%) |
| Otolaryngology | 274 (8.2%) |
| Inpatient | 799 (23.8%) |
| Ophthalmology | 196 (5.8%) |
| Neurology | 509 (15.2%) |
| Plastic surgery | 54 (1.6%) |
| Other specialty | 384 (11.5%) |
| Lesion type† | |
| Cranial nerve neoplasm | 135 (6%) |
| Parotid gland neoplasm | 74 (3.3%) |
| Benign parotid neoplasm | 9 (12.2%) |
| Malignant parotid tumor | 65 (87.8%) |
| Cholesteatoma | 8 (0.4%) |
| Time of lesion diagnosis (months from Bell's palsy diagnosis)* | 12.3 [4.2, 23.8] |
| Receipt of Bell's palsy medication (steroids and antivirals)† | 1250 (32%) |
- * Median (IQR).
- † n (%).
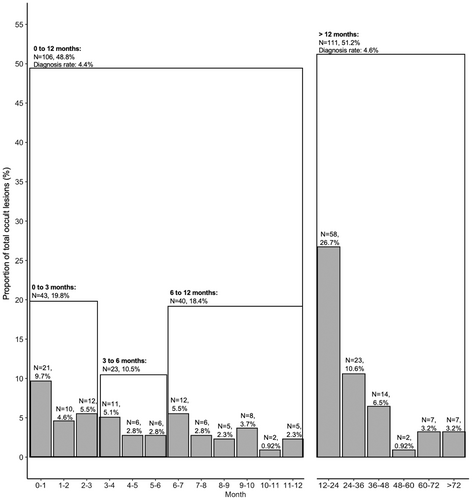
Among the 217 occult lesions diagnosed, 106 (48.8%) had lesion diagnoses within the first 12 months after Bell's palsy diagnosis (Figure 1). Although a large proportion of all occult lesions were diagnosed in the early timeframe of 0–3 months (43 patients [19.8%] at a median of 1.2 [0.4, 2.1] months), 23 (10.6%) and 40 (18.4%) additional individuals were diagnosed with lesions between 3–6 and 6–12 months, respectively, at medians of 4.2 (3.5, 5.1) and 8.4 (6.9, 9.8) months (Table II). Of these occult lesions diagnosed with 12 months, 67% were cranial nerve neoplasms, 29.7% were parotid gland neoplasms, and 2.8% cholesteatomas (Table IIIA). Cranial nerve neoplasm patients were younger than parotid gland neoplasm and cholesteatomas patients (48 vs. 70 and 67, respectively, p < 0.001). Cranial nerve neoplasm patients were also more likely to receive Bell's palsy medication before the lesion diagnosis compared with parotid gland neoplasm patients (31% vs. 9.4%, p = 0.035). Time to lesion diagnosis was not significantly different between the lesion types.
| A. Lesion diagnoses in 0–3 months | B. Lesion diagnoses in 3–6 months | C. Lesion diagnoses in 6–12 months | p value | D. All lesion diagnoses in 0–12 months | E. All lesion diagnoses after 12 months | p value | |
|---|---|---|---|---|---|---|---|
| N | 43 | 23 | 40 | 106 | 111 | ||
| Age* | 54 [43, 70] | 56 [47, 72] | 48 [44, 64] | 0.66 | 54 (43, 67) | 60 (48, 72) | 0.10 |
| Sex† | |||||||
| Female | 22 (51%) | 12 (52%) | 18 (45%) | 0.81 | 52 (49%) | 62 (56%) | 0.3 |
| Male | 21 (49%) | 11 (48%) | 22 (55%) | 54 (51%) | 49 (44%) | ||
| Race† | |||||||
| White | 29 (67%) | 14 (61%) | 30 (75%) | 0.79 | 73 (71%) | 64 (58%) | 0.011* |
| Asian | 2 (4.7%) | 2 (8.7%) | 2 (5.0%) | 6 (5.8%) | 23 (21%) | ||
| Black | 1 (2.3%) | 0 (0%) | 2 (5.0%) | 3 (2.9%) | 1 (0.9%) | ||
| Other | 9 (21%) | 6 (26%) | 6 (15%) | 21 (20%) | 21 (19%) | ||
| Bell's palsy diagnosis site† | |||||||
| Emergency | 4 (21.3%) | 0 (0%) | 1 (2.5%) | >0.9 | 5 (4.7%) | 5 (5.1%) | 0.4 |
| Primary care | 0 (0%) | 1 (12%) | 1 (6.2%) | 2 (1.9%) | 35 (35%) | ||
| Otolaryngology | 13 (81%) | 7 (88%) | 15 (94%) | 35 (34%) | 3 (3.0%) | ||
| Inpatient | 20 (47%) | 8 (38%) | 18 (45%) | 46 (44%) | 3 (3.0%) | ||
| Ophthalmology | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 33 (33%) | ||
| Neurology | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 5 (5.1%) | ||
| Plastic surgery | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 10 (10%) | ||
| Other | 6 (14%) | 5 (24%) | 5 (12%) | 16 (15%) | 17 (15.6%) | ||
| Lesion type† | |||||||
| Cranial nerve neoplasm | 28 (65%) | 16 (70%) | 27 (68%) | 0.81 | 71 (67%) | 64 (58%) | 0.4 |
| Parotid gland neoplasm | 14 (33%) | 7 (30%) | 11 (27%) | 32 (30%) | 42 (38%) | ||
| Cholesteatoma | 1 (2.3%) | 0 (0%) | 2 (5%) | 3 (2.8%) | 5 (4.5%) | ||
| Time of lesion diagnosis (months from Bell's palsy diagnosis)* | 1.17 [0.37, 2.05] | 4.20 [3.50, 5.08] | 8.42 [6.89, 9.84] | <0.001 | 3.8 (1.6, 7.3) | 24 (18, 37) | <0.001 |
| Receipt of Bell's palsy medication (steroids and antivirals)† | 10 (23.2%) | 6 (26%) | 9 (22.5%) | > 0.9 | 25 (24%) | 33 (30%) | 0.3 |
- * Median [IQR].
- † n (%).
| A: Lesions diagnosed within 0–12 months (N = 106) | B: Lesions diagnosed after 12 months (N = 111) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cranial nerve neoplasm | Parotid gland neoplasm | Cholesteatoma | p value | Cranial nerve neoplasm | Parotid gland neoplasm | Cholesteatoma | p value | |
| N | 71 | 32 | 3 | 64 | 42 | 5 | ||
| Age* | 48 (40, 58) | 70 (61, 82) | 67 (57, 74) | <0.001* | 52 (46, 63) | 70 (59, 80) | 65 (54, 67) | <0.001 |
| Gender† | ||||||||
| Female | 35 (49%) | 16 (50%) | 1 (33%) | 0.86 | 45 (70%) | 14 (33%) | 3 (60%) | <0.001 |
| Male | 36 (51%) | 16 (50%) | 2 (67%) | 19 (30%) | 28 (67%) | 2 (40%) | ||
| Race† | ||||||||
| White | 51 (72%) | 20 (62%) | 2 (67%) | 0.35 | 34 (53%) | 28 (67%) | 2 (40%) | 0.4 |
| Black | 0 (0%) | 3 (9.4%) | 0 (0%) | 0 (0%) | 1 (2.4%) | 0 (0%) | ||
| Asian | 5 (7.0%) | 1 (3.1%) | 0 (0%) | 16 (25%) | 6 (14%) | 1 (20%) | ||
| Other | 13 (18%) | 7 (22%) | 1 (33%) | 12 (19%) | 7 (17%) | 2 (40%) | ||
| Bell's palsy diagnosis site† | ||||||||
| Emergency | 3 (4.2%) | 2 (6.5%) | 0 (0%) | <0.001* | 2 (3.4%) | 2 (5.6%) | 1 (25%) | <0.001* |
| Inpatient | 37 (53%) | 9 (29%) | 0 (0%) | 27 (46%) | 8 (22%) | 0 (0%) | ||
| Primary care | 2 (2.9%) | 0 (0%) | 0 (0%) | 1 (1.7%) | 1 (2.8%) | 1 (25%) | ||
| Otolaryngology | 27 (39%) | 5 (16%) | 3 (100%) | 21 (36%) | 10 (28%) | 2 (50%) | ||
| Ophthalmology | 0 (0%) | 0 (0%) | 0 (0%) | 2 (3.4%) | 3 (8.3%) | 0 (0%) | ||
| Neurology | 0 (0%) | 0 (0%) | 0 (0%) | 3 (5.1%) | 0 (0%) | 0 (0%) | ||
| Plastic surgery | 0 (0%) | 0 (0%) | 0 (0%) | 2 (3.4%) | 0 (0%) | 0 (0%) | ||
| Other | 1 (1.4%) | 15 (48%) | 0 (0%) | 1 (1.7%) | 9 (25%) | 0 (0%) | ||
| Time of lesion diagnosis (months from Bell's palsy diagnosis)* | 3.7 [1.4, 7.5] | 3.9 [1.9, 6.8] | 6.8 [4.6, 6.9] | > 0.9 | 21 (16, 31) | 28 (22, 42) | 24 (19, 27) | 0.018* |
| Receipt of Bell's palsy medication (steroids and antivirals)† | 22 (31%) | 3 (9.4%) | 0 (0%) | 0.035* | 18 (28%) | 12 (29%) | 3 (60%) | 0.3 |
- * Median [IQR].
- † n (%).
In the period after 12 months, 111 (51.1%) of the occult lesion cohort received a diagnosis at a median of 23.8 (17.8, 36.8) months after Bell's palsy diagnosis. Cranial nerve neoplasms were the most commonly diagnosed (57.7%), followed by parotid gland neoplasms (37.9%) and cholesteatomas (4.5%) (Table IIIB). Similar to those diagnosed before 12 months, cranial nerve neoplasm patients were the youngest (p < 0.001). Time to lesion diagnosis was different by lesion type (p = 0.018) with parotid gland tumors having the longest diagnosis time of 28 months.
Comparing patients with occult lesions that were diagnosed between 0 and 12 months with those diagnosed after 12 months, the racial distribution was significantly different (p = 0.01) (Table II). Among all Asian patients with lesions, more were diagnosed after 12 months (79.3%) compared with the 0–12-month period (20.7%). In contrast, white patients had similar proportions of lesions diagnosed before (53.3%) after 12 months (46.7%). Age, sex, Bell's palsy diagnosis site, lesion type, and Bell's palsy medication administration did not differ between patients with occult lesions diagnosed before and after 12 months or between lesions diagnosed within the respective timeframes of 0–3, 3–6, and 6–12 months.
In a multivariable regression model assessing whether age, sex, race, and Bell's palsy diagnosis site were associated with lesion detection at any time after Bell's palsy diagnosis, patients aged 40–60 years were more likely to have a lesion diagnosis (OR = 1.57 [1.18–2.09], p = 0.002) compared with patients over 60 (Table IV). Compared with white patients, Black patients had lower odds of lesion diagnosis (OR = 0.32 [1.12–0.7], p = 0.01). Patients who were initially diagnosed with Bell's palsy in an inpatient (OR = 9.64 [5.34–19.2], p < 0.001), otolaryngology (OR = 30.1 [16.3–61.1], p < 0.001), ophthalmology (OR = 3.83 [1.64–9], p = 0.002), or other, unspecified specialty settings (OR = 10.1 [5.10–21.5], p < 0.001), were more likely to have an occult mass discovered compared with patients who were diagnosed in the emergency department. In another similar multivariable regression model assessing factors associated with a lesion diagnosis made at 12 months or later among the 217 patients with lesions, when controlling for age, sex, lesion type, and diagnosis site, only Asian race had higher odds of this later lesion diagnosis (OR = 6.2 [2.23–20.4], p = 0.001) as compared with white race (Table V).
| Characteristic | OR | 95% CI | p value |
|---|---|---|---|
| Sex | |||
| Female (reference) | |||
| Male | 1.02 | 0.78, 1.33 | 0.9 |
| Age group | |||
| > 60 (reference) | |||
| <40 | 0.81 | 0.55, 1.19 | 0.3 |
| 40–60 | 1.57 | 1.18, 2.09 | 0.002* |
| Race | |||
| White (reference) | |||
| Asian | 1.16 | 0.76, 1.74 | 0.5 |
| Black | 0.32 | 0.12, 0.7 | 0.010* |
| Other or mixed | 0.58 | 0.43–0.8 | <0.001* |
| Bell's palsy diagnosis site | |||
| Emergency (reference) | |||
| Inpatient | 9.64 | 5.34, 19.2 | <0.001* |
| Primary care | 1.29 | 0.53, 3.09 | 0.6 |
| Otolaryngology | 30.1 | 16.3, 61.1 | <0.001* |
| Ophthalmology | 3.83 | 1.64, 9.00 | 0.002* |
| Neurology | 1.77 | 0.79, 4.03 | 0.2 |
| Plastic surgery | 3.57 | 0.78, 12.0 | 0.058 |
| Other specialty | 10.1 | 5.10, 21.5 | <0.001* |
- Abbreviation: CI, confidence interval; OR, odds ratio.
| Characteristic | OR | 95% CI | p value |
|---|---|---|---|
| Sex | |||
| Female (reference) | |||
| Male | 0.73 | 0.38, 1.39 | 0.3 |
| Age group | |||
| > 60 (reference) | |||
| < 40 | 0.61 | 0.23, 1.57 | 0.3 |
| 40–60 | 0.84 | 0.40, 1.78 | 0.7 |
| Race | |||
| White (reference) | |||
| Asian | 6.20 | 2.23, 20.4 | 0.001* |
| Black | 1.25 | 0.57, 2.71 | 0.6 |
| Other or mixed | 0.27 | 0.01, 2.75 | 0.3 |
| Lesion type | |||
| Cranial nerve neoplasm (reference) | |||
| Parotid gland neoplasm | 2.01 | 0.90, 4.55 | 0.090 |
| Cholesteatoma | 1.18 | 0.21, 7.21 | 0.8 |
| Bell's palsy diagnosis site | |||
| Emergency (reference) | |||
| Inpatient | 0.40 | 0.07, 1.93 | 0.3 |
| Primary care | 0.85 | 0.07, 10.5 | 0.9 |
| Otolaryngology | 0.47 | 0.08, 2.34 | 0.4 |
| Ophthalmology | 1.33 | 0.13, 15.6 | 0.8 |
| Neurology | 2.13 | 0.09, 6.62 | > 0.9 |
| Plastic surgery | 10.2 | 0.18, 51.1 | > 0.9 |
| Other specialty | 0.24 | 0.03, 1.38 | 0.12 |
- Abbreviation: CI, confidence interval; OR, odds ratio.
DISCUSSION
This study involving over 3,900 facial palsy patients over a 22-year span with 2240 Bell's palsy diagnoses represents one of the largest cohorts of facial and Bell's palsy patients.7, 10, 13-16 We identified 217 (9.7%) patients with occult lesions that were diagnosed at a median of 12.3 (4.2, 23.8) months after Bell's palsy diagnosis and a lesion diagnosis rate of 4.4% within the first 12 months after Bell's palsy diagnosis. These rates parallel data reported by Chung et al., who found a 3.8% occult neoplasm diagnosis rate among a cohort of 240 facial palsy patients over 10 years.15 To our knowledge, we are the first to report a delay in occult lesion diagnosis between Asian and white patients despite no significant differences in lesion types diagnosed between racial groups, highlighting potential racial disparities in timely lesion diagnosis that could be explained by differences in socioeconomic status, cultural barriers, and access to care.17
As facial palsy is the only clinical manifestation of both Bell's palsy and significant manifestation of occult lesions, reaching an accurate diagnosis can be challenging. A large proportion of all lesion diagnoses were made within the 3 months (19.8%), increasing suspicion that the facial palsy was due to the occult mass and not Bell's palsy. For these cases, it is possible that an underlying mass was suspected as the etiology for the facial palsy despite no immediate evidence of the lesion, thus prompting outpatient imaging and evaluation within a short period. However, out of all occult lesions, a total of 151 (69.6%) were diagnosed at least 6 months after Bell's palsy diagnosis at a median of 20 (11, 29) months, representing potential cases of lesion misdiagnosis.
Most existing studies of lesion detection after initial Bell's palsy diagnosis are limited by small cohorts. Among all lesions diagnosed in our cohort, 62.2% (N = 135) were cranial nerve neoplasms, representing 5.6% of the total Bell's palsy cohort. This cranial nerve neoplasm diagnosis rate is higher than national rates; vestibular nerve schwannomas have an annual diagnosis rate of 0.01% in the United States and facial nerve schwannomas are estimated to account for less than 1% of all temporal bone tumors.18, 19 Others have reported similar difficulty in correctly diagnosing occult lesions, with reports of facial schwannoma misdiagnosis ranging from 34% to 62.5%.10, 16, 20 Zhang et al. characterize 11 encounters in which cranial nerve lesions were misdiagnosed and conclude that high misdiagnosis rates can be attributed to the lack of experience with and scarcity of facial nerve lesions.16
Parotid gland neoplasms and cholesteatomas respectively made up 34.1% and 3.7% of all lesions diagnosed after Bell's palsy diagnosis in our cohort. The majority of parotid gland neoplasms detected at any timepoint after a Bell's palsy diagnosis were malignant rather than benign (87.8% vs. 12.2%), which is consistent with the existing literature that malignant parotid gland tumors are more often implicated in causing facial palsy than benign tumors and can manifest as facial palsy in 7%–20% of parotid gland tumor cases.15 Although rare, benign parotid gland neoplasm misdiagnosis rates as Bell's palsy have been described in small case series, because slow growing parotid neoplasms can also create a mass effect in the stylomastoid foramen and compress the facial nerve.21, 22 The rate of occult cholesteatoma masking as Bell's palsy has only been reported in small cohorts.23-26 Facial palsy is a rarer symptom in cholesteatomas, with the prevalence of facial palsy in middle ear cholesteatomas as 3.5% in one study, which parallels our cohort, as 3.7% of occult lesions were cholesteatomas.27
Our findings reveal that compared with patients diagnosed with Bell's palsy in the emergency department, those first diagnosed in inpatient and specialty settings were more likely to have lesion diagnoses. These patients first diagnosed in inpatient or specialty settings might have been more likely to be followed closely, highlighting the utility of following up with an appropriate specialist within the first few months after Bell's palsy diagnosis. It is also possible that although a patient's first diagnosis of Bell's palsy was registered in a specialty setting in our database, these patients were already diagnosed with Bell's palsy in an external community setting and were referred to a specialty clinic due to high suspicion for an occult lesion.
It is well established that delays in diagnosis and treatment initiation in head and neck cancers can result in advanced clinical presentation, leading to limited treatment options and poorer prognoses.12, 28, 29 Falcioni et al. report smaller lesion size as the main predictor of improved postoperative facial nerve function among vestibular schwannoma cases, emphasizing the importance of early detection in functional restoration.30 Among all 217 patients with lesion diagnoses in this cohort, although 43 (19.8%) had lesion diagnoses within the first 3 months, 151 (69.6%) patients received occult lesion diagnoses at least 6 months after Bell's palsy diagnosis at a median of 20 (11, 29) months. This timeframe between Bell's palsy and lesion diagnoses represents a period where earlier recognition and intervention may have improved treatment outcomes, particularly for malignant parotid tumors.
The initial history and a thorough physical exam are central to diagnosing and differentiating an occult mass lesion from Bell's palsy.31 Although imaging is among the first-line diagnostic methods for lesion detection, it is not recommended for Bell's palsy, and a clinical diagnosis is considered sufficient.31 A common MRI finding in acute Bell's palsy is facial nerve labyrinthine segment enhancement, which can persist for several months or longer after symptom onset.32, 33 Notably, there are rare cases of malignancy with perineural invasion that can present simply as facial nerve enhancement without a mass, such as in adenoid cystic carcinoma, which is indistinguishable from typical nerve enhancement.34 Additionally, early occult lesion detection can be difficult with current imaging practices. Although contrast-enhanced MRIs are reliable in detecting parotid gland neoplasms, MRI brain scans in the context of unilateral facial weakness are usually administered to rule out stroke; the scan range for this protocol can often exclude the extratemporal course of the facial nerve, leading to missed parotid gland neoplasm diagnoses.35 Further, although non-contrast MRI is considered sufficient in the workup of acute stroke, contrast significantly improves detection of parotid lesions.35-38 The lack of contrast could increase odds of a missed parotid gland neoplasm or facial nerve schwannoma.
Although imaging is most useful to detect mass lesions, the optimal timing to initiate imaging for an occult mass remains controversial. Zandian et al. and Hohman et al. suggest that Bell's palsy patients with no signs of recovery within 4 months of symptom onset undergo contrast-enhanced imaging, whereas Quesnel et al. recommend waiting at least 6 months for no signs of recovery.9, 13, 14 In contrast, some primary care-based guidelines suggest that patients with gradual symptomatic progression during the first 2–3 weeks after facial palsy onset and lack of significant recovery after 3 months should be imaged, referred, and/or surgically explored.31, 39 Typical cases of Bell's palsy caused by viral neuritis are expected to show signs of symptomatic improvement after 3–4 months. In cases with no signs of recovery by 4 months, there is a greater possibility of an occult malignancy being the actual etiology of the facial palsy; delaying workup of such cases past this timepoint could only delay potential cancer diagnoses.13, 40 The large proportion of occult lesions diagnosed after the initial Bell's palsy diagnosis in our cohort and the overall risks of delayed occult lesion diagnoses underscore the need for careful monitoring for lack of recovery in Bell's palsy. We suggest considering further workup with imaging in Bell's palsy cases with no signs of recovery at 3–4 months, given the natural time course of a true Bell's palsy.
The retrospective, de-identified database limited our ability to clarify clinical details for individual cases. We could not identify the diagnostic methods used to work up facial palsy, which could have contextualized cases where occult lesions were not discovered initially. We also did not have data on the degree of facial weakness at time of diagnosis and information on symptomatic progression that could have prompted follow up visits. Further, as we relied on ICD-10 codes to identify Bell's palsy patients, we must consider the potential issue of miscoding; it is possible that this diagnosis of Bell's palsy was applied in cases where the etiology for a unilateral facial palsy was unclear and needing further workup, even if the presentation did not fit a classic Bell's palsy. Additionally, we could not compare the lesion's sidedness with that of the facial palsy based on the ICD-10 codes. As Bell's palsy is an idiopathic condition with acute onset, it is also possible that a patient independently had both Bell's palsy and a head and neck lesion. However, given the large proportion of lesion diagnoses within close temporal proximity to the initial Bell's palsy diagnosis and the insidious nature of most facial nerve lesions, we believe the vast majority of the cases represented lesions that were initially misdiagnosed as Bell's palsy. Future, prospective studies across multiple institutions are needed to further understand lesion misdiagnosis as Bell's palsy and the clinical consequences of a delayed lesion diagnosis and to increase awareness of occult lesions among all medical specialties treating acute-onset facial palsy.
CONCLUSIONS
Our cohort of 3912 facial palsy patients with 2240 Bell's palsy cases represents one of the largest cohorts of facial and Bell's palsy patients to date. We report a total occult lesion diagnosis rate of 9.7% at a median of 12.3 (4.2, 23.8) months after Bell's palsy diagnosis; 48.8% of occult lesions were diagnosed within the first 12 months. Cranial nerve neoplasms were the most commonly diagnosed (62.2%), followed by parotid gland neoplasms (34.1%) and cholesteatomas (3.7%). This study also highlights racial disparities in timely lesion diagnosis; among all patients with lesions, Asian patients were more likely than white patients to be diagnosed after 12 months compared with first 12 months after Bell's palsy diagnosis. Given the large majority of the total lesions detected within the first 12 months after Bell's palsy diagnosis, our data underscores the importance of timely workup of an occult lesion in cases where the facial palsy fails to recover.




