Depression Symptoms and Olfactory-related Quality of Life
Editor's Note: This Manuscript was accepted for publication on March 22, 2022.
The authors have no funding, financial relationships, or conflicts of interest to disclose.
Abstract
Objectives
Patients with olfactory dysfunction (OD) frequently report symptoms of depression. The objective of this study was to determine how clinical characteristics and olfactory-related quality of life (QoL) measures associate with the likelihood for major depressive disorders (MDDs).
Methods
A total of 192 OD patients were included. Olfactory function was measured using all three subtests of the Sniffn' Sticks test. Olfactory-related quality of life (QoL) was evaluated using the Questionnaires of Olfactory Dysfunction (QOD)-negative (NS) and -positive statement (PS). The likelihood for MDD was assessed using the Patients Health Questionnaire-2 (PHQ-2). Demographics and disease-specific variables (etiology and duration of OD) were collected. Univariate and multivariable analyses were used to associate disease-specific variables and the QOD with the outcome of the PHQ-2. Additionally, the predictive ability of the QOD-NS to predict depressive symptoms was calculated.
Results
In univariate analysis, COVID-19 related smell loss, the QOD-NS, and the QOD-PS were significantly associated with the PHQ-2. In multivariable analyses adjusting for QoL measures, the QOD-NS (ß = 0.532, p < 0.001) and sinonasal OD (compared with postinfectious OD) were significantly associated with the PHQ-2 (ß = 0.146, p = 0.047). When omitting QoL measures from multivariable analyses, only COVID-19 related OD (compared with postinfectious OD) was significantly associated with the PHQ-2 (ß = 0.287, p = 0.009). A QOD-NS score > 20.5 had 70.13% sensitivity and 76.32% specificity for detecting symptoms of depression.
Conclusion
Our results suggest that COVID-19 related OD might be associated with a higher likelihood for MDD. Furthermore, we showed that the QOD-NS score might be helpful to predict symptoms of depression in OD patients.
Level of Evidence
4 Laryngoscope, 132:1829–1834, 2022
INTRODUCTION
Smell loss is a common disease that significantly impacts affected individuals' quality of life (QoL) and has emerged as a public health concern during the coronavirus disease 2019 (COVID-19) pandemic.1-3 The causes of olfactory dysfunction (OD) are diverse, including head traumas, upper respiratory tract infections, or exposure to toxins. Smell loss can also develop secondary to neurodegenerative and sinonasal diseases, such as chronic rhinosinusitis (CRS).4 Although smell loss has been linked to major depressive disorders (MDD),5, 6 the associations between olfactory-related QoL measures and the risk for depressive symptoms have only been evaluated in CRS patients and OD patients in general.7-9 Identifying associations with MDD in specific causes of smell loss is essential to understand its key drivers further and improve the clinical interpretability of commonly used patient-reported outcome measures (PROMs).
Various PROMs, such as the Questionnaire of Olfactory Dysfunction (QOD), have been developed to quantify smell loss symptoms' effect on patients' QoL.8, 10 The QOD itself is one of the most widely used olfactory-specific measurements. Although initially developed as a 52-item questionnaire, it has been shortened due to reliability issues.11 It is currently primarily used as the 17-item QOD-negative statement (QOD-NS) that evaluates the impact of smell loss on daily life and the 2-item QOD-positive statement (QOD-PS) that assesses the ability of patients to cope with OD.2, 3, 7, 11-13
To further understand the development of depression in OD patients, we must first determine which clinical characteristics or OD symptoms are the primary drivers of MDD. In this study, we hypothesize that specific clinical characteristics (such as the reason for smell loss) and olfactory-related QoL measures would differentially associate with the likelihood of depressive symptoms. We, therefore, sought to determine associations between clinical characteristics and olfactory-related QoL measures with the likelihood of MDD in a cohort of patients with various causes of smell loss.
MATERIAL AND METHODS
Subjects
This was a retrospective study carried out at the outpatient clinic for Smell and Taste Disorders of the Department of Otorhinolaryngology, Head and Neck Surgery, Medical University of Vienna. The study protocol was approved by the Ethical Scientific Committee of the Medical University of Vienna (1984/2021). All subjects visited our outpatient clinic between April 2019 and October 2021 with the main complaint of subjective olfactory dysfunction. All patients underwent thorough history taking, standardized ear, nose, and throat examination (including nasal endoscopy), completed validated patient-reported outcome measures (PROMs), and received comprehensive olfactory testing. Patients were asked to report a history of diabetes or hypertension. Smoking history was stratified as nonsmoker, former smoker, and current smoker. The suspected underlying etiology of smell loss was diagnosed based on the current “European Position paper of olfactory dysfunction.”4 All COVID-19 patients were laboratory-confirmed (polymerase chain reaction-based or antibody test) and diagnosed starting from the first confirmed COVID-19 case in Austria (February 25, 2020).
Olfactory testing
Quantitative olfactory performance was assessed based on the Sniffin' Sticks test (Burghart Messtechnik, Holm, Germany), which measures olfactory function based on three subdimensions: odor threshold (T), discrimination (D), and identification (I). The exact test procedure is described in detail elsewhere.14, 15 We classified our patients using published normative datasets and defined olfactory performance as follows: (i) normosmia (TDI ≥ 30.75), (ii) hyposmia (TDI < 30.75 and >16), and (iii) anosmia (TDI ≤ 16).16, 17
Patient-reported outcome measures
To quantify the olfactory-specific QoL, all patients completed the validated 19-item QOD.8, 12 The 19-item QOD consists of two subcategories, the QOD-NS with 17-items and the QOD-PS with two items. While the QOD-NS evaluates the negative impact of smell loss on QoL, the QOD-PS assesses the ability of patients to cope with smell loss. The QOD items are based on a Likert scale ranging from 0 (I disagree) to 3 (I agree). Therefore, higher QOD-NS scores represent lower olfactory-related QoL, whereas higher QOD-PS scores represent a better adjustment to OD.
To evaluate the risk for MDDs in our patients with OD, we used the German version of the Patient Health Questionnaire 2 (PHQ-2), a two-item PROM that was developed as a screening tool. The PHQ-2 consists of two questions: “Over the last two weeks, how often have you been bothered by the following problems (1) Little interest or pleasure in doing things and (2) Feeling down, depressed, or hopeless?” which are scored on a Likert scale ranging from 0 (not at all) to 3 (nearly every day).18, 19 A higher PHQ-2 represents a higher likelihood of suffering from MDD.
Statistical analysis
We performed statistical analysis and data visualization using GraphPad Prism 9.1.0 (GraphPad Software, Inc., La Jolla, CA) and SPSS 26.0 (IBM Corp., Armonk, NY). Continuous data are presented as mean ± standard deviation, whereas categorical data are presented as absolute numbers (%). We used univariate and multivariable linear regression analysis to evaluate the associations between clinical characteristics: age (in years), gender (reference: female), smoking status (nonsmoker compared with former and current smoker, reference: nonsmoker), duration of smell loss (in months), olfactory function (TDI score), the reason for smell loss (postinfectious, posttraumatic, idiopathic, sinonasal, and COVID-19 related smell loss, reference: postinfectious OD), and health-related PROMs (QOD-NS and QOD-PS) with the outcome of the PHQ-2 score. To evaluate the diagnostic accuracy of the QOD-NS score in identifying patients who are at a higher risk for MDD (as represented by a PHQ-2 score ≥ 219), we computed receiver operating characteristics (ROC) curves and the area under the ROC curves (AUC). The level of significance was set at 0.05.
RESULTS
Patient demographics
We included 192 patients with subjective smell loss (62.5% female, mean age ± SD = 47.8 ± 18.8 years). The biggest group of our patients was classified as COVID-19 related OD (n = 53), followed by idiopathic (n = 52), postinfectious (n = 40), posttraumatic (n = 18), sinonasal (n = 13), iatrogenic (n = 6), and toxic (n = 4) smell loss. We also included three cases of neurodegenerative and three cases of congenital OD. The mean ± SD duration of smell loss was 32.4 ± 42.5 months, excluding our congenital cases (Table I). Quantitative testing of olfactory function using the Sniffin' Sticks TDI test revealed that most patients were hyposmic (n = 106), followed by anosmic (n = 66), and normosmic patients (n = 20). We also included patients with olfactory test scores within the normosmic range as we wanted to include all patients with subjective OD.
| Patients with Smell Loss (n = 192) | |
|---|---|
| Age in years, mean (SD) | 47.8 (18.8) |
| Gender (N) | 120F, 72M |
| Duration of smell loss in months, mean (SD) | 32.4 (42.5) |
| Diabetes mellitus | 19 (9.9%) |
| Arterial hypertension | 5 (2.6%) |
| Smoking status | |
| Current | 34 (17.71%) |
| Former | 56 (29.2%) |
| Nonsmoker | 102 (53.1%) |
| Olfactory Function | |
|---|---|
| Sniffin' Sticks TDI Test, mean (SD) | 20.6 (8.1) |
| Threshold, mean (SD) | 3.6 (2.4) |
| Discrimination, mean (SD) | 9.1 (3.2) |
| Identification, mean (SD) | 8.0 (3.8) |
| Olfactory function | |
| Hyposmics | 106 (55.2%) |
| Anosmics | 66 (34.4%) |
| Normosmics | 20 (10.4%) |
| Reason for Smell Dysfunction | |
|---|---|
| Postinfectious | 40 (20.8%) |
| Posttraumatic | 18 (9.4%) |
| Idiopathic | 52 (27.1%) |
| COVID-19 | 53 (27.6%) |
| Iatrogen | 6 (3.1%) |
| Toxic | 4 (2.1%) |
| Sinonasal | 13 (6.8%) |
| Congenital | 3 (1.6%) |
| Neurodegenerative | 3 (1.6%) |
| Patient-Reported Outcome Measures | |
|---|---|
| QOD-NS, mean (SD) | 19.1 (11.5) |
| QOD-PS, mean (SD) | 3.3 (2.0) |
| PHQ-2, mean (SD) | 1.5 (1.8) |
- Continuous data are presented as mean (standard deviation). Categorical data are presented as number (%).
Differences in depressed mood between different etiologies of smell loss
Previous studies provided evidence that depressed mood might be modulated by the degree (i.e., anosmia and hyposmia) and the reason for smell loss.11, 20, 21 Therefore, we first sought to evaluate whether there were differences in the PHQ-2 score (i) between the anosmic, hyposmic, and normosmic patients and (ii) between the patients with postinfectious, posttraumatic idiopathic, sinonasal, and COVID-19 related smell loss. We excluded our iatrogenic, toxic, neurodegenerative, and congenital cases from group comparisons due to the small number of patients.
One-way ANOVA revealed no significant difference in PHQ-2 score between our normosmic, hyposmic, and anosmic patients [F(2, 189) = 1.243, p = 0.290]. On the contrary, one-way ANOVA revealed a significant difference in PHQ-2 score between our postinfectious, posttraumatic, idiopathic, sinonasal, and COVID-19 related OD patients [F(4, 171) = 2.563, p = 0.0402]. Tukey's post hoc test revealed a significantly higher PHQ-2 score in patients with COVID-19 related smell loss than in those with postinfectious OD (p = 0.0196) (Fig. 1).
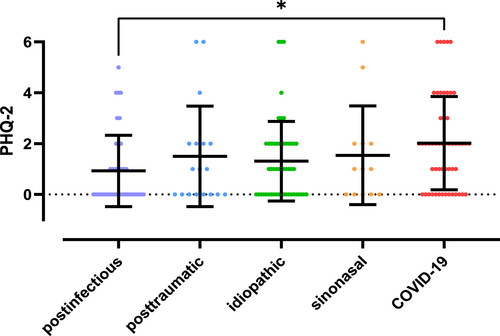
Olfactory-related QoL is differentially associated with the likelihood for MDD
In the next step, we were interested to know whether the olfactory-related QoL, as represented by the QOD-NS (negative impact of smell loss in everyday life) and the QOD-PS (the ability to cope with OD), were associated with the likelihood for MDD, as represented by the PHQ-2. We performed univariate and multivariable linear regression analysis with the outcome of PHQ-2 while also controlling for clinical characteristics such as demographics (age and gender), smoking status (nonsmoker compared with former and current smoker), duration of smell loss (in months), olfactory function (TDI score), and reason for smell loss (postinfectious, posttraumatic, idiopathic, and COVID-19-related).
In univariate analysis, we found a significant positive association between COVID-19 related smell loss and the PHQ-2 (ß = 0.209, 95% CI = 0.063–0.356, p = 0.005). Furthermore, we also found a significant positive association between the QOD-NS and the PHQ-2 (ß = 0.532, 95% CI = 0.406–0.659, p < 0.001), indicating that a lower olfactory-related QoL was associated with a higher likelihood for depressive symptoms. We also found that the QOD-PS was significantly negatively associated with the PHQ-2 (ß = −0.261, 95% CI = −0.406 to −0.117, p < 0.001), indicating a higher ability to cope with OD was associated with a lower likelihood for MDD. We found no relevant association between demographics and clinical characteristics with the PHQ-2. In multivariable analyses, the QOD-NS (ß = 0.547, 95% CI = 0.403–0.690, p < 0.001) and sinonasal smell loss (ß = 0.146, 95% CI = −0.002 to 0.290, p = 0.047) were significantly associated with the PHQ-2 (Table II).
| Univariate Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| β (95% CI) | p value | β (95% CI) | p value | |
| Age (in years) | −0.120 (−0.269 to 0.028) | 0.112 | 0.137 (−0.016 to 0.290) | 0.078 |
| Gender (female) | −0.120 (−0.269 to 0.029) | 0.113 | 0.002 (−0.134 to 0.138) | 0.978 |
| Smoking status (nonsmoker) | 0.069 (−0.081 to 0.218) | 0.366 | 0.123 (−0.004 to 0.251) | 0.057 |
| Duration of smell loss (in months) | −0.145 (−0.293 to 0.004) | 0.056 | −0.029 (−0.179 to 0.121) | 0.702 |
| Etiology of smell loss | ||||
| Postinfectious | Reference | — | Reference | — |
| Posttraumatic | 0.006 (−0.144 to 0.155) | 0.941 | −0.006 (−0.160 to 0.148) | 0.939 |
| Idiopathic | −0.062 (−0.211 to 0.087) | 0.415 | 0.026 (−0.142 to 0.194) | 0.759 |
| Sinonasal | 0.011 (−0.139 to 0.161) | 0.885 | 0.146 (0.002 to 0.290) | 0.047 |
| COVID-19 | 0.209 (0.063 to 0.356) | 0.005 | 0.113 (−0.075 to 0.301) | 0.238 |
| Olfactory function (TDI) | 0.032 (−0.117 to 0.182) | 0.670 | 0.001 (−0.144 to 0.146) | 0.989 |
| QOD-NS | 0.532 (0.406 to 0.659) | <0.001 | 0.547 (0.403 to 0.690) | <0.001 |
| QOD-PS | −0.261 (−0.406 to −0.117) | <0.001 | −0.137 (−0.276 to -0.002) | 0.054 |
- ß, Linear regression coefficient.
- Statistical significance is set at p < .05.
The reason for smell loss is associated with the likelihood for MDD
As we found that the reason for smell loss was significantly associated with the likelihood for MDD in univariate analysis, we were also interested to know which clinical characteristics were most strongly associated with the likelihood for MDD when omitting the olfactory-related QoL. We omitted health-related outcome measures from these multivariable analyses to report clinically more relevant results as the QOD is not yet used as a standardized QoL questionnaire for OD patients worldwide.4, 10
Multivariable analyses revealed that only COVID-19 related smell loss was significantly positively associated with the PHQ-2 score (ß = 0.287, 95% CI = 0.072 to 0.502, p = 0.009) (Table III).
| Multivariable Analysis | ||
|---|---|---|
| β (95% CI) | p value | |
| Age (in years) | 0.004 (−0.170 to 0.179) | 0.961 |
| Gender (female) | −0.116 (−0.269 to 0.038) | 0.140 |
| Smoking status (nonsmoker) | 0.100 (−0.049 to 0.249) | 0.186 |
| Duration of smell loss (in months) | −0.111 (−0.282 to 0.060) | 0.202 |
| Etiology of smell loss | ||
| Postinfectious | Reference | - |
| Posttraumatic | 0.121 (−0.055 to 0.297) | 0.178 |
| Idiopathic | 0.133 (−0.061 to 0.326) | 0.178 |
| Sinonasal | 0.133 (−0.035 to 0.302) | 0.121 |
| COVID-19 | 0.287 (0.072 to 0.502) | 0.009 |
| Olfactory function (TDI) | −0.052 (−0.221 to 0.249) | 0.546 |
- ß, Linear regression coefficient.
- Statistical significance is set at p < .05.
The QOD-NS score is an accurate indicator for a higher risk of MDD
In the last step, we wanted to know whether the QOD-NS score is an accurate indicator for a higher risk of MDDs, defined as a PHQ-2 score ≥ 219 by calculating a ROC curve.
The AUC of 0.790 (95% CI = 0.722–0886, p < 0.001) indicates a good diagnostic accuracy of the QOD-NS to identify those at a higher risk for MDD. The QOD-NS cutoff of >20.5 maximized the sum of sensitivity and specificity to detect patients with smell loss that score ≥2 on the PHQ-2 with a sensitivity of 70.13% and a specificity of 76.32% (Fig. 2).
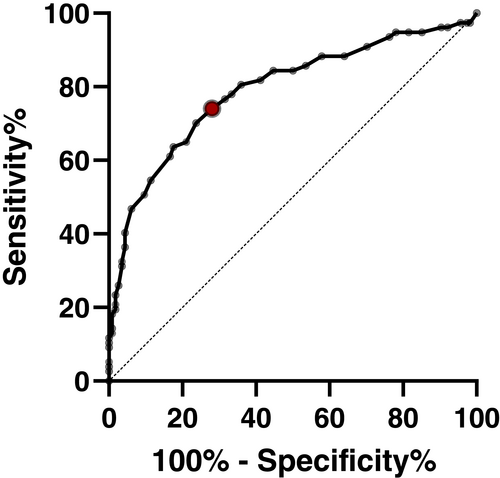
DISCUSSION
Olfactory dysfunction is associated with a significant health-related QoL detriment.1 The clinical manifestation of the disease is not only associated with a decrease in general health-related QoL but also in olfactory-specific QoL.8, 22 Considering the close relationship between poor health-related QoL and more significant depressive symptoms,23 it is not surprising that MDDs have already been associated with smell loss.5, 6 Previous studies provided the first evidence for a positive association between olfactory-related QoL measures and the risk for depressive symptoms in CRS (in which smell loss is reported in up to 80% of patients24) and OD patients in general.7-9 We, therefore, hypothesized that olfactory-related QoL could also be a dominant determinant of a higher likelihood for depressive symptoms in OD patients. In this study, we explored the effects of clinical characteristics and olfactory-related QoL on the likelihood of MDD in a large cohort of patients with different causes of smell loss. In our analysis including olfactory-related QoL measures in multivariable analyses, we found that the QOD-NS was positively associated with the PHQ-2, which indicates that a higher burden of smell loss in daily life was associated with a higher likelihood for MDD. Similarly, we found that sinonasal smell loss was positively associated with the PHQ-2, indicating that sinonasal smell loss (compared to postinfectious OD) might be associated with a higher likelihood for MDD. In a subanalysis omitting all QoL measures from multivariable analyses, we found that only COVID-19 related OD (compared to postinfectious OD) was significantly associated with the likelihood of MDD. Lastly, the analysis also revealed that the QOD-NS score (representing QoL detriments related to smell loss) is an accurate indicator for higher risk of depressive symptoms.
Patient-reported outcome measures gained significant importance in routine clinical care to improve patient engagement, shared decision making, and self-management.25 For patients with OD, the recent “Position Paper on Olfactory Dysfunction”4 recommends using validated questionnaires in the clinical assessment. Similarly, a recent review on PROMs related to smell loss also concluded that a questionnaire might be a time-efficient and elegant method to evaluate the patient's perspective of the dysfunction.10 Previous studies in a general cohort of patients with smell loss showed that the original QOD-NS (39-items) correlated with the Beck's Depression Inventory,26 a PROM that evaluates the severity of depression. In contrast, the original QOD-PS (5-items) showed no relevant correlations.8 Similarly, the refined QOD-NS (17-item) has also correlated with the PHQ-2 in patients with CRS.7 Our study shows that measurements of olfactory-related QoL differentially associate with the likelihood for MDD in a large cohort of OD patients with different etiologies, including COVID-19 related smell loss. Specifically, we found that the 17-item QOD-NS was associated with the PHQ-2 in univariate and multivariable analyses. We found that the QOD-NS showed a positive association with the PHQ-2, indicating that lower olfactory-related QoL associates with a higher likelihood for MDD. We also found a negative association between the QOD-PS and the PHQ-2 in univariate analysis, indicating that better coping strategies might be associated with a lower likelihood for MDD in patients with OD. This finding was not surprising, considering the well-known negative relationship between positive coping strategies and the likelihood of depressive symptoms.27, 28 Since only a small number of long-term OD patients regain normal age-related olfactory function over time, one might reasonably suggest that the majority of smell loss patients adjust to and develop coping strategies to cope with smell loss.29
Past studies have shown that smell loss, in general, is associated with depression.5, 6, 8, 11, 20 However, whether the etiology of smell loss is also associated with the likelihood for MDD was unclear. In this study, we present the novel finding that the sinonasal and COVID-19 smell loss groups might be the most vulnerable groups concerning depressive symptoms. We have previously shown that the individual significance of olfaction decreases with the duration of smell loss.30 Therefore, one might suggest that the duration of smell loss might be associated with a lower likelihood for MDD since the importance of olfaction becomes lower over time. However, univariate and multivariable analyses revealed no relevant association between the duration of smell loss and the PHQ-2, but only an association with the sinonasal and COVID-19 OD groups. One explanation why sinonasal smell loss (compared with postinfectious OD) was positively associated with the likelihood of MDD in multivariable analyses while controlling for olfactory-related QoL might relate to the underlying chronic inflammatory disease, which is known to not only impact olfactory function and the nasal symptoms, but various other sinonasal subdomains such as sleep, otologic/facial pain, and emotional function.31, 32 Interestingly, the prevalence of MDD in CRS was proposed to be higher than that of the general population.33 Similarly, the positive association between COVID-19 smell loss (compared to postinfectious OD) and MDD when omitting olfactory-related QoL measures from analysis might relate to extensive post-COVID conditions that reduce the QoL of affected individuals. Indeed, previous studies provided the first evidence that depressive symptoms are commonly reported in patients recovering from COVID-19.34-36
Our findings may also have potentially important implications for evaluating patients with smell loss. As the relationship between QoL and depression is increasingly found to be co-dependent, our results provide a means for assessing depression symptoms in OD patients by querying for MDD in those with lower olfactory-related QoL. This might also require the routine use of validated QoL measures during the clinical assessment of patients with smell loss. Previous studies in patients with CRS have shown that those with comorbid depression might not achieve equivalent long-term results after treatment than those without MDD.33 It is possible that patients with smell loss and comorbid depression might also recover less than those without MDD. Therefore, future studies should investigate the impact of comorbid depression on treatment outcomes in OD patients undergoing olfactory training.
This study included a large cohort of patients with various causes of smell loss. Nonetheless, results should be interpreted within the context of the limitations. Firstly, we only used a screening tool, the PHQ-2, to evaluate the likelihood for MDD, but not the former diagnosis of depression, for which the “gold standard” is currently believed to be individual clinical interviews.37 Secondly, this was a cross-sectional study, while a longitudinal study might have allowed us to evaluate the impact of depressive symptoms on QoL changes in OD patients. Nevertheless, we believe that our results are important to pave the way for future studies that further explore the impact of smell loss and comorbid MDD on health- and disease-specific QoL and explore its associations with treatment outcomes.
CONCLUSION
This study adds to the current literature on clinical olfactory research in two important ways. First, we found associations of QoL measures with the likelihood for MDD, suggesting that a lower olfactory-related QoL might also impact the likelihood for depressive symptoms in OD patients. Second, it provides the first evidence that the QOD-NS can also be used to screen for smell loss patients at a higher risk for MDD.
ACKNOWLEDGMENTS
We thank Verena Niebauer, Johannes Raninger, Stefan Kovacs, and Peter Boga for their help with data collection.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interest regarding the publication of this paper.




