Cochlear pharmacokinetics of cisplatin: An in vivo study in the guinea pig
This work was supported by AFA Insurance, Foundation Tysta Skolan, and Acta Oto-Laryngologica. Training of the surgical procedure and protocol for sampling of ST perilymph from apex was kindly provided by Professor Alec N. Salt, Washington University, St. Louis, U.S.A. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Abstract
Objectives/Hypothesis
Cisplatin produces toxic lesions to outer hair cells (OHCs) in the cochlear base but not in the apex. The objective of this study was to compare the pharmacokinetic profile of cisplatin in scala tympani (ST) perilymph in the cochlear base and apex, respectively.
Study Design
In vivo animal study.
Methods
Forty-seven guinea pigs were given an intravenous bolus injection of an ototoxic dose of cisplatin. Ten to 240 minutes after cisplatin was given, blood, cerebrospinal fluid (CSF), and ST perilymph were aspirated within the same target time. ST perilymph was aspirated from the basal turn and from the apex of the cochlea by two different sampling techniques. Liquid chromatography with postcolumn derivatization was used for quantitative determination of the parent drug.
Results
Ten minutes after administration, the concentration of cisplatin in ST perilymph was 4-fold higher in the basal turn of the cochlea than in the apex. At 30 minutes, the drug concentrations did not differ. At 60 minutes, the level of cisplatin in ST perilymph and blood UF was equivalent. The perilymph-blood ratio increased thereafter with time.
Conclusion
The pharmacokinetic findings of an early high concentration of cisplatin in the base of the cochlea and delayed elimination of cisplatin from ST perilymph compared to blood might correlate to the cisplatin-induced loss of OHCs in the base of the cochlea.
Level of Evidence
N/A. Laryngoscope, 123:3172–3177, 2013
INTRODUCTION
Cisplatin is a platinum-containing anticancer drug used in the treatment of a number of solid malignant neoplasms in adults and is also given to children with medulloblastoma and nephroblastoma.1 The major antineoplastic effect of cisplatin is its binding to DNA and the inhibition of cell replication.2 Cisplatin has two major dose-limiting side effects: nephrotoxicity and ototoxicity.3 Although cisplatin ototoxicity is a relatively research-intensive area, no protective measures are available for the hearing function in connection with cisplatin-based chemotherapy. The ototoxic side effects of cisplatin are dose-dependent with an obvious interindividual variability.4, 5 If severe hearing loss is diagnosed, cisplatin is often excluded from further therapy, which might reduce the effect of the antineoplastic treatment.6, 7 Cisplatin treatment mainly affects the high frequencies of hearing, and damage is related to outer hair cells (OHCs) in the base of the cochlea.8, 9 Since OHCs are nonreplicating cells, the mechanism of apoptosis has been directed to DNA-independent mechanisms.10 Cisplatin generates reactive oxygen species (ROS) that seem to be involved in the ototoxic process.11 It also has an active biotransformation product, the monohydrated complex (MHC) that is reported to be more cytotoxic12 and also more ototoxic than cisplatin.13 Studies showing the importance of cisplatin concentrations in cochlear fluids in relation of the ototoxic effects are rare.14, 15
In the present study, an ototoxic dose of cisplatin was given by intravenous (i.v.) injection to guinea pigs in order to study the pharmacokinetics of the drug in the cochlea. The study had three main objectives: 1) investigate the pharmacokinetics of cisplatin in ST perilymph in the cochlear base and apex and compare these findings to the distribution of cisplatin in blood and cerebrospinal fluid (CSF); 2) assess the elimination rate of cisplatin in ST perilymph; and 3) analyze MHC in ST perilymph.
MATERIALS AND METHODS
Animals
Forty-seven female guinea pigs (range 246–500 g), Duncun-Hartley, (Lidköpings kaninfarm AB, Sweden) were used. The animals were housed at the animal facility at Karolinska University Hospital and were kept in enrichment cages at a room temperature of 21°C, with a 12-hour light–dark cycle and free access to food and tap water. During all surgical procedures the guinea pigs were placed on a Harvard homoeothermic surgical table with a rectal temperature of 38°C. At the conclusion of the experiment, the animals were decapitated while still under anaesthesia. Care and use of the animals reported in this study was approved in accordance with ethical standards at Karolinska Institutet and was consistent with national regulations for care and use of the animal (Ethical permits N 372/08).
Experimental Design
The main objective was to aspirate ST perilymph, blood, and CSF from every animal within the same target time. The mean concentration of cisplatin was plotted versus the mean actual sampling time. Animals were divided into three groups according to the site and time of sampling ST perilymph: Group Ia (n = 15) from the base of the cochlea; group Ib (n = 15) from the cochlear apex 10–30 minutes after i.v. injection of cisplatin; and group II (n = 17) from the base of the cochlea 30–240 minutes after cisplatin administration.
Anaesthesia
Animals in groups Ia and II were anesthetized with a combination of an intramuscular injection of ketamine and xylazine, as described previously.15 A different anaesthetic procedure was used for the animals in group Ib in order to maintain an adequate anaesthesia and oxygenation. Animals in group Ib were supplied with a tracheostomy and anaesthetized with a combination of fentanyl and fluanisone (Hypnorm, VetaPharma Ltd, Leeds, UK) and midazolam (Midazolam Panpharma, PharmaPlus, Oslo, Norway). The ventrolateral surgical approach to get access to the cochlear apex requires longer surgical time and the animal has to lie on its back throughout the experiment. Atropine was given subcutaneously to reduce mucus in the airways and bupivacaine was administrated for local anaesthesia. Animals in group Ia and Ib were supplied with an external flow of oxygen.
Cisplatin
Cisplatin (Platinol 1 mg/ml, Bristol-Myers Squibb Pharmaceuticals, New York) 8 mg/kg body weight was given intravenously to all animals at a rate of 1 ml/minute.
Surgical Procedure
The animal was attached to a head holder. The right jugular vein was catheterized along the venous flow toward the heart and used for i.v. administration of cisplatin. The left jugular vein was catheterized against the venous flow and used for blood sampling. A dorsolateral surgical approach described previously15 was used to access the basal turn of the cochlea of both ears of group Ia and II animals. In animals belonging to group Ib, a ventrolateral surgical approach was used to access the apical part of the right cochlea. After blunt dissection medial to the mandible, the digastric muscle was reduced. The bulla bone was opened, thereby exposing the apical part of the cochlea. The construction of a silicone cup on the apical part of the cochlea was previously described.16 Clear ST perilymph was slowly filling up the silicone cup.
Sampling Procedure
Calibrated capillary tubes were used to collect ST perilymph. One capillary was used to aspirate 1 μl ST perilymph from the basal turn of the left and right cochlea respectively, and 10 different capillaries were used for sequential sampling (numbered 1–10) of 1 μl ST perilymph from the apex of the right cochlea. The total volume of ST perilymph in guinea pig cochlea is calculated to be around 4.7 μl.17, 18 Sequential samples 4 and 5 were thereby aspirated within approximately the same target time as ST perilymph was sampled from the basal turn of animals in group Ia. Due to an open cochlear aqueduct, the last sequential samples, that is, 8 to 10, are known to be highly contaminated with CSF.19 After ST perilymph was collected, one sample of 0.35 ml blood was aspirated from the left jugular vein. An additional sample of 0.35 ml blood was aspirated at another target time from animals in group Ia. CSF was the last sample to be taken. A sharp syringe was used for the suboccipital puncture, and about 10 μl clear CSF was aspirated from the cistern magna.20
Sample Analysis
Samples were handled and stored as described previously.15 Liquid chromatography with postcolumn derivatization was used to determine the concentration of cisplatin and to analyze the monohydrated complex of cisplatin, MHC.21 All samples were stored at −80°C until analysis, which occurred within 3 weeks.
Statistical Analysis
For descriptive data mean ± SD was used. Two-tailed nonparametric (Mann-Whitney) test was used to compare two independent groups. Differences for which P values were 0.05 or less were considered to be statistically significant.
RESULTS
Pharmacokinetics of Cisplatin in Blood Ultrafiltrate (blood-UF), CSF, and ST Perilymph During 10 to 30 Minutes After Drug Administration
The mean time to aspirate 1 μl of ST perilymph from the cochlear base was about 30 seconds. The distribution phase of cisplatin to the base of the cochlea was fast, with the highest concentration of cisplatin obtained at the first target time. Figure 1 shows the pharmacokinetic profile and Table 1 summarizes the concentrations of cisplatin in blood-UF, CSF, and ST perilymph 10 to 30 minutes after i.v. injection. The maximum concentration of cisplatin in blood-UF is seen at 10 minutes, and after 30 minutes there is a 42% reduction. The highest level of cisplatin in ST perilymph aspirated from the base of the cochlea is also seen at the first target time. The concentration of cisplatin in the cochlear apex is calculated from the mean of the first sequential sample. At 10 minutes there is a significantly lower concentration in apex compared to the base (P = 0.02). Cisplatin in the apex increases significantly already at 20 minutes (P = 0.03). No difference in cisplatin concentration in the cochlear base and apex can be observed at 30 minutes (P = 0.79). The concentration of cisplatin is significantly lower in CSF compared to ST perilymph aspirated from the base of the cochlea at all target times. Cisplatin concentrations in blood-UF and CSF did not differ between animals in group Ia and Ib (only significant different cisplatin concentrations in blood-UF at 20 min.). This indicates that the different surgical and anesthetic procedures would not affect the cochlear pharmacokinetics.
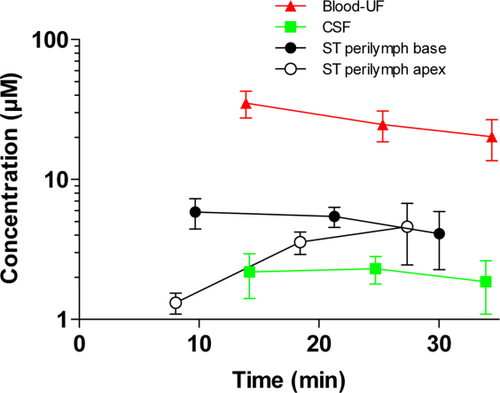
Pharmacokinetic profile of cisplatin in blood-UF, CSF, and ST perilymph in the base and apex of the cochlea 10 to 30 minutes after cisplatin (8 mg/kg) was given i.v. The numbers of analyzed samples per time point are shown below. Red-filled triangles show the concentration in blood-UF (Ia + Ib, n = 9–12). Green-filled squares show the concentration in CSF (Ia + Ib, n = 9–11). Black-filled circles show the concentration in ST perilymph from the basal turn of the left and right cochlea (Ia, n = 5). And black open circles show the concentration in ST perilymph from sequential sample number 1 from the apex of the right cochlea (Ib, n = 3–5). Data are the mean; error bars are ± SD. CSF = cerebrospinal fluid; i.v. = intravenous; SD = standard deviation; ST = scala tympani ; UF = ultrafiltrate. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
| 10 minutes | 20 minutes | 30 minutes | ||||
|---|---|---|---|---|---|---|
| Concentration (μM) | Time (minutes) | Concentration (μM) | Time (minutes) | Concentration (μM) | Time (minutes) | |
| Blood-UF | 35.10 ± 7.60 | 13.90 ± 2.09 | 24.70 ± 6.10 | 25.28 ± 1.95 | 20.20 ± 6.60 | 34.48 ± 1.88 |
| CSF | 2.18 ± 0.77 | 14.20 ± 1.85 | 2.30 ± 0.51 | 24.71 ± 2.35 | 1.86 ± 0.77 | 33.89 ± 1.03 |
| ST perilymph base | 5.86 ± 1.43 | 9.68 ± 0.93 | 5.44 ± 0.89 | 21.27 ± 0.98 | 4.09 ± 1.82 | 30.03 ± 1.23 |
| ST perilymph apex (capillary 1) | 1.32 ± 0.23 | 8.00 ± 0.72 | 3.56 ± 0.65 | 18.50 ± 0.60 | 4.60 ± 2.15 | 27.30 ± 1.24 |
| ST perilymph (capillary 4–5) | 1.92 ± 0.47 | 9.96 ± 0.49 | 3.54 ± 0.51 | 20.07 ± 0.31 | 3.96 ± 1.50 | 28.75 ± 0.51 |
| ST perilymph (capillary 8–10) | 2.20 ± 0.70 | 12.72 ± 1.91 | 2.51 ± 0.19 | 22.57 ± 1.33 | 3.58 ± 1.10 | 30.92 ± 1.09 |
- Data are mean ± standard deviation.
- CSF= cerebrospinal fluid; i.v. = intravenous; ST = scala tympani ; UF = ultrafiltrate.
Sequential Samples 1 to 10 From Cochlear Apex During 10 to 30 Minutes
Figure 2 shows a more detailed presentation of the pharmacokinetic profile of cisplatin in the sequential perilymph samples aspirated from the cochlear apex. The time for sampling capillaries 1 through 10 was 5.08 ± 1.26 minutes. At 10 minutes there is a significant difference in concentration gradient between sample number 1 and the mean of sample numbers 4 and 5 (P = 0.02). This gradient is absent at 20 and 30 minutes (P = 0.98, P = 0.57, respectively). The last sequential samples 8 through 10 have an equivalent concentration of cisplatin, as shown in CSF aspirated from the same animals in group Ib at 10 and 20 minutes (P = 0.89, P = 0.34, respectively).
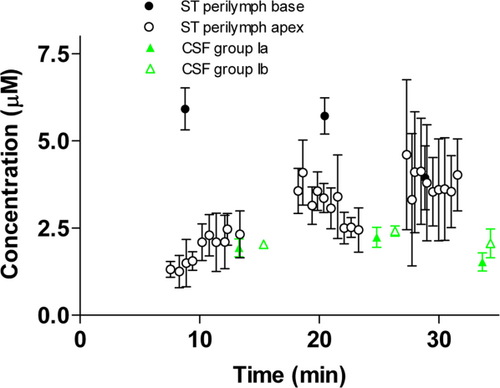
Pharmacokinetics of cisplatin in sequential samples number 1 to 10 of ST perilymph from the cochlear apex 10 to 30 minutes after cisplatin (8 mg/kg) was given i.v. The numbers of analyzed samples per time point are shown below. Black-filled circles show the concentration in ST perilymph from the basal turn of the left and right cochlea (Ia, n = 5). Open circles show the concentration in ST perilymph from sequential samples number 1–10 from the apex of the right cochlea (Ib, n = 3–5). Green-filled triangles show the concentration in CSF from animals in group Ia (n = 5). Green open triangles show the concentration in CSF from animals in group Ib (n = 4–5). Data are the mean, error bars are ± SD. CSF = cerebrospinal fluid; i.v. = intravenous; SD = standard deviation; ST = scala tympani. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Mean of Sequential Samples 4 and 5 Versus ST Perilymph Aspirated Directly From the Basal Turn
The concentration of cisplatin in the base of the cochlea was compared between the mean level of cisplatin in sequential samples 4 and 5 aspirated from the cochlear apex and ST perilymph aspirated directly from the basal turn. A significant difference in concentration is seen at 10 and 20 minutes (P = 0.01, P = 0.02, respectively). No difference is seen at 30 minutes (P > 0.99).
Pharmacokinetics of Cisplatin in Blood-UF, CSF, and ST Perilymph During 30 to 120 Minutes After Drug Injection
Late phase changes in cisplatin concentration demonstrate delayed elimination of cisplatin from the base of the cochlea. Figure 3 shows the pharmacokinetic profile of cisplatin in blood-UF, CSF, and ST perilymph aspirated from the basal turn of the right cochlea 30 to 120 minutes after cisplatin was given. At 60 minutes, the levels of cisplatin in ST perilymph and blood-UF are equivalent. Figure 4 shows an increase in ST perilymph/blood-UF ratio of cisplatin after 60 minutes, displaying slow elimination of cisplatin from ST perilymph. The ratio between ST perilymph and blood-UF concentration increases from 0.4 to 5.8 during the sampling period (30–120 min.).
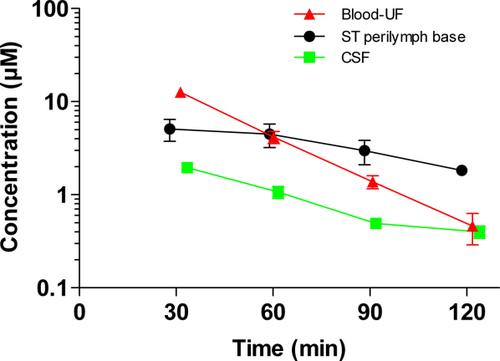
Pharmacokinetic profile of cisplatin in blood-UF, CSF, and ST perilymph in the base of the cochlea 30 to 120 minutes after cisplatin (8 mg/kg) was given i.v. to guinea pigs in group II. The numbers of analyzed samples per time point are indicated. Red-filled triangles show the concentration in blood-UF (n = 4–8). Green-filled squares show the concentration in CSF (n = 2–5). And black-filled circles show the concentration in ST perilymph from the basal turn of the right cochlea (n = 2–4). Data are the mean, error bars are ± SD. CSF = cerebrospinal fluid; i.v. = intravenous; SD = standard deviation; ST = scala tympani; UF = ultrafiltrate. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
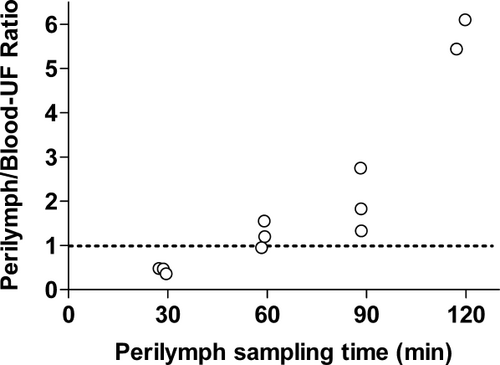
Ratio of the concentration of cisplatin in ST perilymph from the basal turn of the cochlea compared to blood-UF 30 to 120 minutes after cisplatin was given i.v. At 60 minutes, the concentrations of cisplatin in ST perilymph and blood-UF were similar. i.v. = intravenous; ST = scala tympani ; UF = ultrafiltrate.
Analysis of MHC in ST Perilymph
Seventeen samples of ST perilymph aspirated from the basal turn of the left cochlea of group II animals were analyzed for the concentration of MHC. All samples had a concentration of MHC below the level of detection (1 μM).
DISCUSSION
The ototoxic effect of cisplatin is characterized by a gradient of lesions to target cells along the cochlear duct, with the greatest injuries seen in the OHCs in the base of the cochlea. A number of factors have been attributed to this longitudinal pattern, which is seen in both human temporal bones and cochleae from experimental animals exposed to the drug.22 In the present study, an ototoxic dose of cisplatin was given i.v. to guinea pigs. Cisplatin could be analyzed in blood-UF, CSF, and ST perilymph up to 120 minutes after treatment. Ten minutes after its administration, a significant higher concentration of cisplatin was found in ST perilymph sampled from the base of the cochlea as compared to apical aliquots. It was also found that cisplatin had a delayed elimination in ST perilymph compared to blood. These two findings might influence drug transport in the inner ear into deeper cochlear compartments, and thereby be of importance for the ototoxic effect of cisplatin. Similar pharmacokinetic studies have been performed on gentamicin due to its ototoxic side effect. Gentamicin also shows rapid uptake, early saturation, and long exposure to the cochlear tissue.23, 24
When cisplatin is given i.v., a number of well-defined as well as unknown mechanisms contribute to its final distribution to the target cells in the cochlea. Cisplatin binds strongly to plasma proteins, whereby a large part of it becomes nonactive.25 The nonbound level of cisplatin analyzed in this study represents the active portion of the drug.26 Cisplatin's relatively high concentration in blood within the early phase after drug administration might favor the transport of cisplatin to the cochlea. Little is known about drug transport to and within the cochlea, but it is clear that the accessibility of the inner ear is limited by two barrier systems: the blood-perilymph barrier and the intrastrial fluid-blood barrier,27 that is, the interface between blood and endolymph.28, 29 The intrastrial fluid-blood barrier30 is maintained by the endothelial cells sealed by tight junctions, as well as by the cochlear pericytes and perivascular resident macrophage-type melanocytes. Nevertheless, it is well-established that a higher concentration of cisplatin is obtained in perilymph than in endolymph after systemic administration.31 It is not known how cisplatin enters the perilymphatic and endolymphatic compartments, but both passive diffusion and active transport via transport proteins have been discussed.22, 32 The production of the perilymphatic fluid as well as the permeability of the intrastrial fluid-blood barrier is still not fully identified.33, 34 Indirect evidence for the importance of drug concentration in the perilymphatic compartment for the ototoxic process of cisplatin was obtained in a recent study comparing it with the mainly non-ototoxic third-generation platinum compound oxaliplatin.15
In the present study, the pharmacokinetic profile of cisplatin in ST perilymph along the cochlear duct is shown for the first time. By using two different techniques to sample ST perilymph, cisplatin could be analyzed both from the basal turn and the apex of the cochlea within approximately the same target time. Enhanced early transport to the basal ST perilymph was displayed; the concentration of cisplatin in the ST perilymph at the base of the cochlea was more than four-fold higher than the concentration of cisplatin in apical samples at 10 minutes. This difference might reflect a difference in uptake of cisplatin from blood to ST perilymph, that is, transport mechanisms involving a blood-perilymph barrier along the cochlear duct. After 30 minutes, an equivalent concentration of cisplatin was seen in the base and apex of the cochlea. The susceptibility of OHCs in the base of the cochlea to ototoxic drugs can be multifactorial, involving reduced cytoplasmic detoxification and decreased tolerance to DNA- or non-DNA–dependent toxic mechanisms.35, 36 Most likely, the regulation of cisplatin uptake in different parts of the cochlea is more complex and requires additional mechanisms.
With the use of a sequential sampling technique to aspirate ST perilymph from the apex of the cochlea, a gradient of cisplatin in ST perilymph could be further studied during the distribution phase to the deeper compartments of the cochlea. At 10 minutes, the sequential samples demonstrated a base-to-apex gradient of cisplatin, that is, the mean of sequential sample numbers 4 and 5 representing ST perilymph from the basal part of the cochlea showed a significantly higher concentration of cisplatin compared to the first sequential sample representing ST perilymph in the apex of the cochlea. A limitation of the study was the obvious risk of CSF contamination to the ST perilymph aspirated from the base of the cochlea. It has been documented that 1 μl of ST-perilymph aspirated from the basal turn has a 15% contamination of CSF.37 This study, which shows a lower concentration of cisplatin in CSF compared to ST perilymph sampled from the basal turn, excludes a false, too high concentration of cisplatin in the cisplatin basal ST perilymph results.
It can be speculated, if the initial high concentration of cisplatin in the basal turn that gives a longer exposition time to high levels of the drug might favor the loss of OHC in the base of the cochlea. This observation is in line with the results reported after local administration of cisplatin, where an initial high concentration of cisplatin in the base of the cochlea is related to the ototoxic side effect of cisplatin.38, 39 A pronounced loss of OHCs has predominantly been demonstrated in the base of the cochlea, and thereby lager threshold shifts are observed at the higher frequencies.39 The mechanism for uptake of cisplatin to the OHCs is not yet identified. The basolateral parts of the OHCs are connected to the perilymphatic compartment,40 and the uptake of cisplatin to OHCs is believed in part to be mediated via transport proteins.22 The most studied transport proteins in relation to cisplatin ototoxicity are the membrane protein organic cation transporters (OCTs)41 and the copper transporter (Ctrl).42, 43 In an attempt to display the concentration of the more toxic biotransformation product MHC in ST perilymph, a total of 17 samples were analyzed. The concentration of MCH in ST perilymph was below the level of detection in all samples. This outcome is not unexpected as MHC has earlier been analyzed in blood after the same dose of cisplatin was given to guinea pigs displaying a MHC concentration of 8% to 9% of the total amount of cisplatin in blood.44, 45 Environmental factors such as a low chloride concentration favor the formation of MHC.25 ST perilymph has an chloride concentration and a pH46 equivalent to blood, indicating that formation of MHC in perilymph would be similar as in the blood compartment. Nevertheless, it cannot be excluded that even the low level of MHC in ST perilymph can be involved in the ototoxic side effect of cisplatin.
Researchers have attempted to increase our knowledge regarding hearing protection during cisplatin administration, but no clinical otoprotective treatment has really succeeded.47, 48 The ideal treatment would act specifically against ototoxicity without interfering with the antitumor effect of cisplatin. Such treatment might be given locally to the inner ear, and several ways of administration have been evaluated.49 Two findings from the present study can be of interest for developing a novel therapeutic approach to protect against cisplatin ototoxicity. First, OHCs in the base of the cochlea have a longer exposition time to high concentrations of cisplatin compared to OHCs in the apex. Second, elimination of cisplatin from ST perilymph is slower compared to the clearance of the drug from the blood.
CONCLUSION
The concentration of cisplatin in the base of the cochlea was four-fold higher than the concentration in the apex within the first 10 minutes of cisplatin injection. At 30 minutes, the concentrations in the base and apex were similar. Cisplatin could be analyzed in ST perilymph up to 120 minutes after administration, and delayed elimination of the drug from ST perilymph compared to blood was observed.
ACKNOWLEDGEMENT
Thanks to Professor Staffan Eksborg, Karolinska Institutet, Stockholm, Sweden for editing the figures.




