Evodiamine inhibits EPRS expression to regulate glutamate metabolism and proliferation of oral squamous cell carcinoma cells
Abstract
The effects of evodiamine (EVO) on oral squamous cell carcinoma (OSCC) are not yet understood. Based on our earlier findings, we hypothesized that evodiamine may affect OSCC cell proliferation and glutamate metabolism by modulating the expression of EPRS (glutamyl-prolyl-tRNA synthetase 1). From GEPIA, we obtained EPRS expression data in patients with OSCC as well as survival prognosis data. An animal model using Cal27 cells in BALB/c nude mice was established. The expression of EPRS was assessed by immunofluorescence, Western blotting, and quantitative PCR. Glutamate measurements were performed to evaluate the impact of evodiamine on glutamate metabolism of Cal27 and SAS tumor cells. transient transfection techniques were used to knock down and modulate EPRS in these cells. EPRS is expressed at higher levels in OSCC than in normal tissues, and it predicts poor prognosis in patients. In a nude mouse xenograft model, evodiamine inhibited tumor growth and the expression of EPRS. Evodiamine impacted cell proliferation, glutamine metabolism, and EPRS expression on Cal27 and SAS cell lines. In EPRS knockdown cell lines, both cell proliferation and glutamine metabolism are suppressed. EPRS's overexpression partially restores evodiamine's inhibitory effects on cell proliferation and glutamine metabolism. This study provides crucial experimental evidence supporting the potential therapeutic application of evodiamine in treating OSCC. Evodiamine exhibits promising anti-tumor effects by targeting EPRS to regulate glutamate metabolism.
1 INTRODUCTION
Oral squamous cell carcinoma (OSCC) stands as one of the most prevalent malignancies within the head and neck region. The global toll of 170,000 deaths and over 370,000 new cases reported in the latest Global Cancer Statistics underlines its severity.1 Despite some strides in cancer treatment research, the prognosis for OSCC patients remains grim, with a 5-year survival rate below 50%.2 Urgent exploration of novel therapeutic strategies is imperative. Recent years have witnessed growing interest in natural compounds derived from traditional Chinese medicine due to their potential anticancer properties.3 Among these, evodiamine has emerged as a widely studied natural compound, showcasing significant efficacy in inhibiting tumor growth.4
Evodiamine, a natural indole alkaloid extracted from the Evodia ruticarpa plant, has demonstrated potent anticancer activity against a range of tumors.5 It exhibits topoisomerase inhibition6 and has been shown to impact tumor characteristics like apoptosis,7 cell proliferation,8 invasion, migration,9, 10 and so forth. Metabolic reprogramming is a hallmark of tumorigenesis,11 encompassing alterations in processes like aerobic glycolysis, the citric acid cycle, one-carbon metabolism, and lipid metabolism.12 Among these, amino acid metabolism plays an indispensable role in deciphering the growth characteristics of tumors.13 However, whether evodiamine can modulate tumor amino acid metabolism and elucidate the mechanisms behind its anticancer effects remains a subject with limited in-depth research and confirmation.
Tumors adapt to their environment through reprogramming, with amino acid metabolism emerging as a key player in supporting survival,14 drug resistance,15 and proliferation. While normal cells typically require amino acids at relatively low levels, cancer cells demand substantial amounts to sustain their uncontrolled growth.16 Aminoacyl-tRNA synthetases (aaRS) are pivotal in amino acid metabolism, catalyzing the transfer of amino acids to their cognate tRNAs (charging process), thus priming them for subsequent protein synthesis.17, 18
EPRS, a member of the aaRS family, is responsible for catalyzing the connection of two amino acids, glutamate and proline, to their homologous tRNA, which is essential for subsequent protein synthesis. Currently, EPRS has been linked to various diseases and conditions.19 A deficiency in EPRS can induce integrated stress response and promote apoptosis, which is associated with the occurrence and poor prognosis of multiple myeloma.17 It has also been reported to influence gastric cancer cell proliferation and tumor growth by mediating the WNT/GSK-3β/β-catenin signaling pathway.20 Therefore, EPRS likely plays a crucial role in protein synthesis in cancer development, although further investigation is needed to confirm this.
Against this backdrop, our research group conducted a series of preliminary studies,21 including the application of RNA-seq technology. Through RNA-seq sequencing and analysis, we acquired comprehensive mRNA expression data of OSCC cells post-evodiamine treatment. This dataset provided vital clues for probing the impact of evodiamine on cellular metabolism. Building upon these preliminary investigations, our current study seeks to uncover the mechanistic insights into evodiamine's role in cellular amino acid metabolism.
In this study, we initially employed gene expression profiling interactive analysis (GEPIA) and the human protein atlas (HPA) database to scrutinize the correlation between EPRS and OSCC patients. Subsequently, using both cellular and animal models, we validated EPRS as a prospective target for evodiamine-based OSCC therapy. Our investigation sheds light on how evodiamine influences glutamate metabolism and, in turn, impacts the proliferation of oral squamous cell carcinoma cells.
2 MATERIALS AND METHODS
2.1 Materials
Evodiamine was supplied by MedChemExpress (Shanghai, China) and was dissolved in dimethyl sulfoxide DMSO (stored at − 20 °C). Primary antibodies against β-Actin (Cat. 81,115-1-RR), and EPRS (Cat.67712-1-Ig) were supplied by Proteintech (Wuhan, China). HRP secondary antibody (Cat.GB23301) was supplied by Servicebio (China). FITC*Goat Anti Mouse IgG(H + L) was supplied by Immunoway (USA).
2.2 Cell lines and cell culture
The immortalized oral squamous cell carcinoma cell line Cal27 and SAS were purchased from the (American Type Culture Collection, Manassas, VA, USA). Cal27 was cultured in DMEM (Servicebio, China) with 10% fetal bovine serum and 1% P/S in standard conditions at 37°C and 5% CO2. SAS were cultured in DMEM/F12 (Servicebio, China), and other conditions were the same as Cal27.
2.3 Cell transfection
HieffTrans liposomal nucleic acid transfection reagents were purchased from Yeaseen (China). siEPRS was ordered from GenePharma (Shanghai, China). The pCMV-EPRS overexpression plasmid was purchased from Hewu Technology (Shanghai, China). EPRS knockdown was achieved by transfecting siEPRS, and EPRS overexpression was achieved by transfecting pCMV-EPRS. Cells were seeded in 6-well plates, and when the cell density reached 90%–95%, they were transfected with 20 μM siEPRS using HieffTrans transfection reagent for Cal27 and SAS cells to knock down EPRS. The same conditions were used to transfect Cal27 and SAS cells with 2 μg of pCMV-EPRS for EPRS overexpression.
2.4 RNA isolation and qRT-PCR
RNA was extracted from cells or tissues using a Trizol reagent. Subsequently, reverse transcription of RNA was performed using X PrimeScript RT Master Mix (for Real Time) (Takara Bio, Japan) to synthesize cDNA. Following that, qPCR was conducted using TB Green Premix Ex Taq II (Takara Bio, Japan). mRNA expression levels were normalized to the expression level of β-actin. The results were analyzed using the 2-ΔΔCT method, with three replicates for each sample. The primer sequences were as follows: EPRS f: ACGCTTTCCAACAGTAAGAGGCGTT; EPRS r: ACAAACATTGGGAA-GTAGCAGTTTTCAACA; β-actin-f CTCCATCCTGGCCTCGCTGT; β-actin-r: GCTGTCACCTTCACCGTTCC (synthesized by Sangon Biotech, Shanghai, China).
2.5 Protein extraction, protein concentration measurement, and Western blotting
Cells were scraped from the wells of a culture plate using mechanical means and then lysed on ice with RIPA buffer containing PMSF. The lysates were sonicated, followed by centrifugation to obtain the supernatant. The protein mass and concentration were determined using a BCA assay kit (Solarbio, China). Subsequently, SDS-PAGE gel electrophoresis was performed, and after completion, proteins were transferred to a PVDF membrane. β-actin was used as an internal reference. Protein bands were detected using an ECL chemiluminescent reagent.
2.6 Glutamate content assay
According to the manufacturer's instructions, cellular glutamate content was measured using a glutamate assay kit (Solarbio, China). First, cells were collected and centrifuged into a microcentrifuge tube. 1 mL of reagent was added for every 10 million cells or 0.1 g of tissue. The samples were sonicated, centrifuged at 10,000 rpm for 10 min, and the supernatant was collected. Absorbance was measured at 340 nm using a spectrophotometer at 20 s (A1) and 5 min 20 s (A2). ΔA = A2 − A1, and compared to a standard curve to calculate glutamate content.
2.7 Immunohistochemistry
The complete mouse tumor tissue was carefully dissected and washed with saline, then placed in tissue fixation solution (4% paraformaldehyde). After 48 h of fixation, the tissue was trimmed as needed, dehydrated using a graded alcohol series, cleared in xylene, and embedded in paraffin. Sections of 4 μm were obtained using a microtome. After dewaxing and drying, the sections were rehydrated with ethanol and subjected to antigen retrieval using citrate buffer. The sections were thoroughly rinsed with PBS, blocked with goat serum working solution, and then incubated overnight with the primary antibody at 4°C. After that, the sections were incubated with the secondary antibody for 2 h, followed by incubation with horseradish peroxidase-conjugated streptavidin for 10 min. Finally, the sections were counterstained with hematoxylin and observed under a microscope, and images were captured.
2.8 Tumor xenograft studies
Animal experiments were conducted in strict accordance with the guidelines for the care and use of laboratory animals. BALB/c nude mice (females, 5 weeks old, weighing 16–20 g) were procured from Spfbiotech (Beijing, China) and were raised under specific-pathogen-free conditions. Cal27 xenografts were established in female BALB/c nude mice, and tumor growth was allowed for over 1 week until tumors were palpable using calipers. Subsequently, the mice were treated with 10 or 20 mg/kg of evodiamine via oral gavage.
All experimental procedures were carried out with the approval of the Laboratory Animal Ethics Committee of the Stomatology Department at Lanzhou University.
2.9 Bioinformatics analysis
Differentially expressed gene (DEG) data were sourced from previous publications by our research group. Amino acid pathway information was obtained from Wikipathway (https://www.wikipathways.org) and Reactome (https://reactome.org). Predictive results were generated using an online Venn diagram tool (https://bioinformatics.psb.ugent.be/webtools/Venn/). Clinical correlations of EPRS were assessed using the online resources Gepia (http://gepia.cancer-pku.cn) and the HPA database (https://www.proteinatlas.org).
2.10 Statistical analysis
All data in this study are presented as the mean ± SD of three independent experiments. Differences between groups were determined by one-way analysis of variance using Graph Pad Prism 9, followed by Bonferroni post hoc tests for multiple comparisons. Results were considered statistically significant when p< 0.05 in this study.
3 RESULTS
3.1 EPRS exhibits elevated expression in OSCC and predicts an unfavorable prognosis
To investigate the impact of evodiamine on amino acid metabolism in OSCC, we performed RNA-seq analysis on Cal27 cells treated with evodiamine.21 Our goal was to identify key molecules affected by evodiamine by analyzing changes in mRNA levels. Subsequently, we identified 102 DEGs based on expression differences and reliability. Comparing DEGs with the amino acid metabolism pathway (WP3925,22 R-HAS-7129123) (Figure 1A), we unveiled three genes that might be involved in amino acid metabolism. Notably, EPRS exhibited significantly elevated expression in OSCC compared to normal tissues (Figure 1B), with levels gradually increasing along with tumor pathological stages (Figure 1C). Subsequently, mining the GEIPA database24 revealed that patients with lower EPRS expression displayed markedly better overall survival, whereas those with higher EPRS expression exhibited poorer survival rates (Figure 1D). Hence, we delved deeper into the functional role of EPRS in amino acid-related pathways, discovering its significance in glutamate metabolism. EPRS, a pivotal molecule in the glutamate metabolic pathway, was observed to process glutamate and form Glu-tRNA, essential for subsequent protein synthesis (Figure 1E). Furthermore, analysis in the HPA database highlighted that EPRS protein expression in normal tissues was considerably lower than its expression in tumor tissues (Figure 1F), corroborating our mRNA-level observations.
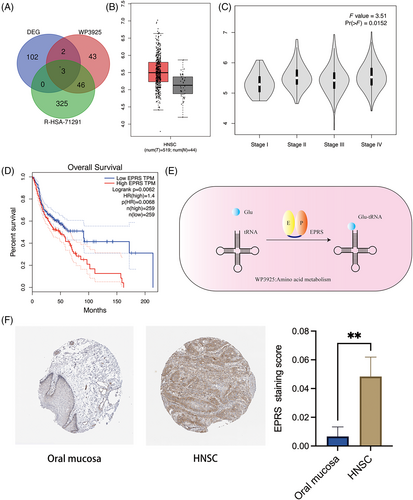
Collectively, our findings underscore that EPRS is highly expressed in OSCC patients and its elevated expression predicts an unfavorable prognosis in OSCC.
3.2 In mice models, evodiamine inhibited tumor glutamate metabolism involving EPRS and positively impacted tumor prognosis
To further validate the impact of evodiamine on OSCC glutamate metabolism, we established an OSCC nude mouse model. We administered evodiamine orally through gavage (once every 2 days for a total of 14 days) to observe changes in tumor development (Figure 2A). Subsequently, we observed that the body weight of mice in the control group and the 10 mg/kg evodiamine group did not change significantly, while the mice in the 20 mg/kg evodiamine group showed weight loss (Figure 2B). Importantly, after 14 days of treatment, we observed a trend of tumor size reduction with increasing concentrations of evodiamine (Figure 2C).
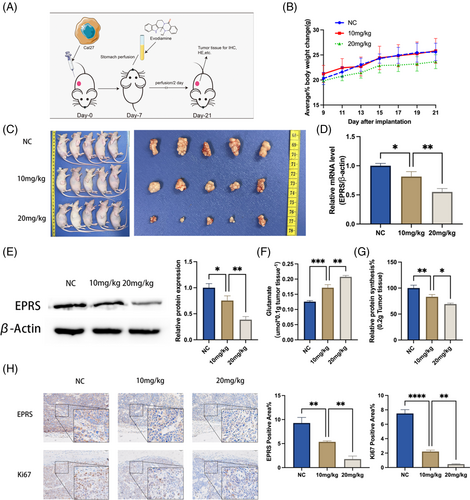
To investigate the mechanism further, we isolated tumor tissues from the mice and extracted RNA from the tissues for qPCR experiments. The results showed that EPRS expression gradually decreased with increasing concentrations of evodiamine (Figure 2D). This result was further confirmed by Western blotting experiments, which showed a decrease in EPRS expression in tumor tissues with increasing concentrations of evodiamine (Figure 2E).
Combining our understanding of EPRS's functional role in the glutamate metabolism pathway, we conducted measurements of free glutamate and total protein content in tumor tissues. The results showed that as EPRS expression decreased, free glutamate levels increased (Figure 2F), while total protein synthesis decreased (Figure 2G). Additionally, we performed immunohistochemistry experiments to detect the expression of the tumor prognosis marker Ki67.25 We found that as EPRS expression decreased, the Ki67-positive area also showed a decreasing trend (Figure 2H).
In summary, evodiamine can inhibit glutamate metabolism processes involving EPRS and improve tumor prognosis in vivo.
3.3 Evodiamine inhibits OSCC cell EPRS-mediated glutamate metabolism and cell proliferation activity
Based on the results of animal experiments, we hypothesize that evodiamine may inhibit Glu-tRNA synthesis and cell proliferation activity in OSCC cells through EPRS. Therefore, we conducted the following experiments using OSCC cell lines Cal27 and SAS.
To assess the effect of Evodiamine on cell proliferation, we first performed a CCK-8 assay, which showed that evodiamine effectively inhibited cell proliferation (with increased inhibition over time and concentration). The IC50 values for Cal27 and SAS at 48 h were 9.657 and 14.36 μM (Figure 3A), respectively, with Cal27 being more sensitive to evodiamine. These conditions were used for subsequent evodiamine treatment of Cal27 and SAS cells. Then, in colony formation assays, we found that evodiamine could inhibit the colony-forming ability of tumor cells (Figure 3B).
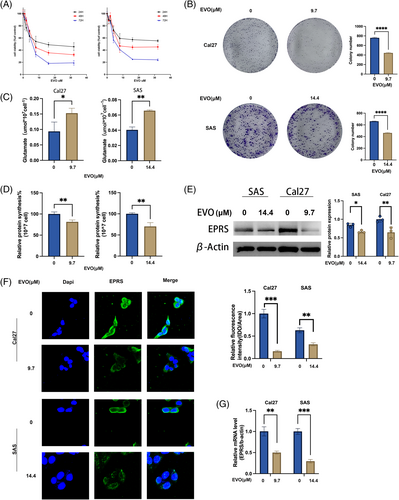
To assess the impact of EVO on glutamate metabolism, we measured the levels of free glutamate in treated cells, revealing an increase in free glutamate upon treatment with evodiamine (Figure 3C). Additionally, through BCA protein assays, we observed a reduction in total protein synthesis in cells treated with evodiamine (Figure 3D).
Finally, to further investigate the molecular mechanisms underlying evodiamine's effects on glutamate metabolism, we conducted qPCR and WB experiments to assess EPRS expression. The qPCR results showed that EPRS relative expression in the evodiamine-treated group was lower than in the control group (Figure 3G). WB results demonstrated a significant decrease in EPRS expression in the evodiamine-treated group (Figure 3E). IF results showed that after evodiamine treatment, EPRS expression in the cytoplasm of Cal27 and SAS cells was reduced (Figure 3F).
These data collectively suggest that evodiamine can influence the metabolic process of EPRS-mediated glutamate synthesis protein and inhibit tumor cell proliferation activity.
3.4 Knocking down EPRS in oral squamous cell carcinoma (OSCC) cells suppressed the utilization of glutamate and cell proliferation activity
To investigate the impact of EPRS on glutamate metabolism in OSCC cells, we planned to knock down the expression of EPRS in OSCC cells and observe changes in glutamate metabolism and cell proliferation. We used the transfection reagent, Hieff Trans®siRNA/miRNA Transfection Reagent, to transfect the corresponding siRNA, interfering with the expression of EPRS in OSCC cells (Figure 4A). We then validated the knockdown efficiency of siRNA through qPCR experiments and found that siEPRS#1292 had the best knockdown efficiency for Cal27 cells, while siEPRS#3676 had the best knockdown efficiency for SAS cells, so we used these two siRNAs as experimental conditions for subsequent experiments (Figure 4B).
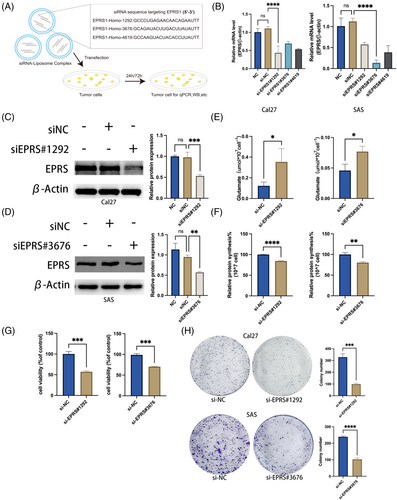
Next, we verified the protein expression efficiency of EPRS through WB experiments and found that EPRS expression was significantly reduced in Cal27 and SAS cells after the respective siRNA treatments (Figure 4C, D), indicating successful knockdown of EPRS.
To investigate the changes in glutamate metabolism in OSCC cells after EPRS knockdown, we first measured the levels of free glutamate in EPRS knockdown cells and found an increase in glutamate levels (Figure 4E). We then detected the total protein content in the cells through BCA assays and observed a decrease in total protein content in the knockdown cells (Figure 4F). The increase in glutamate levels and the decrease in total protein synthesis were consistent with the expected trend of EPRS knockdown.
To further explore the impact of EPRS on cell proliferation, we performed CCK8 and colony formation assays. We found that EPRS knockdown significantly inhibited cell proliferation (Figure 4G, H).
These results indicate that EPRS knockdown leads to a reduction in total protein synthesis utilizing glutamate and inhibits cell proliferation in oral squamous cell carcinoma cells.
3.5 Evodiamine inhibits the synthesis of cellular proteins and the proliferation activity of OSCC by targeting EPRS
To investigate the impact of evodiamine on the proliferative activity of OSCC cells by targeting EPRS, we designed a plasmid, pCMV-EPRS1, for EPRS overexpression (Figure 5A), which was subsequently used for transfection. Through qPCR experiments, we verified the transfection efficiency of pCMV-EPRS1 and observed a significant increase in cellular EPRS expression post-transfection, indicating successful EPRS overexpression. Simultaneously, we treated the overexpressed EPRS cells with evodiamine and found a noticeable reduction in the inhibitory effect of evodiamine on EPRS expression (Figure 5B, C). Subsequently, WB conducted experiments (Figure 5D, E), which yielded results consistent with the qPCR findings, confirming successful EPRS overexpression in OSCC cells.
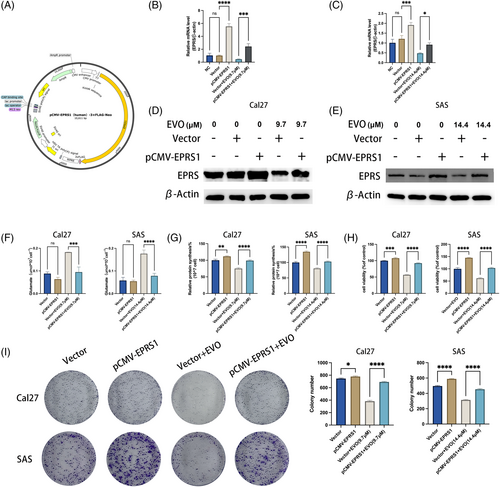
To further investigate the impact of EPRS overexpression on OSCC cells, we initially measured the levels of glutamate and total protein in the cells. We observed a significant decrease in intracellular glutamate content and a slight increase in total protein content after EPRS overexpression, with reduced inhibition by evodiamine on total protein synthesis (Figure 5F, G). Additionally, regarding cell proliferation, we conducted CCK8 assays and colony formation assays, revealing an enhanced capacity for cell proliferation and colony formation following EPRS overexpression. Moreover, Cal27 and SAS cells overexpressing EPRS displayed increased resistance to the growth-inhibitory effects of evodiamine (EVO) (Figure 5H, I).
In summary, our experimental results suggest that evodiamine inhibits Glu-tRNA synthesis protein and proliferation activity in OSCC cells by targeting EPRS overexpression.
4 DISCUSSION
In this research, we investigated the mechanism through which evodiamine inhibits the proliferation of OSCC cells. Firstly, the phenomena that EPRS (as one of the DEGs of Cal27 treated with evodiamine) is overexpressed in OSCC compared to normal tissue, and high EPRS expression is associated with poor prognosis in OSCC were found. Then, in a nude mouse OSCC xenograft model and cell models (Cal27, SAS) treated with evodiamine, suppression of EPRS expression and cell proliferation has been observed. Additionally, EPRS were identified to regulate OSCC cell proliferation through the experiment of EPRS knockdown in Cal27 and SAS cells. Finally, in a rescue experiment, Cal27 and SAS cells were transfected with the pCMV-EPRS1 plasmid to overexpress EPRS. It was observed that EPRS overexpression could reverse the inhibitory effect of evodiamine on cell proliferation. This study elucidates the role of EPRS in the anti-proliferative effect of evodiamine in OSCC and provides strong evidence for EPRS as a target for the anti-tumor action of evodiamine.
Metabolic reprogramming is a prominent feature of cancer. In recent years, research on traditional Chinese medicine and its components targeting metabolic reprogramming has garnered widespread attention. Amino acid metabolism, as a process that connects with other metabolic pathways, plays a crucial role in the initiation and progression of malignant tumors.26 Therefore, targeting amino acid metabolism is considered a potential therapeutic approach for cancer.27 Some traditional Chinese medicines achieve their anti-tumor effects by modulating amino acid metabolism, examples of which include Physalis pubescens L.,28 Glycyrrhiza glabra,29 Hedyotis diffusa,30 and others. Furthermore, evodiamine, a traditional Chinese medicine, has gained significant attention for its anti-tumor properties in the past two decades. Initially, it was discovered that evodiamine could intercalate into DNA and inhibit Topoisomerase 1 (Top1) and Topoisomerase 2 (Top2), making it effective against cancer.31 Subsequently, more research has emerged on the mechanisms by which evodiamine functions in cancer treatment. This includes blocking the cell cycle,32 promoting apoptosis,33 activating relevant signaling pathways,34 and inhibiting tumor metastasis,35 among other effects. Therefore, we were interested in whether evodiamine targets amino acid metabolism reprogramming to exert its anti-tumor effects. However, as of now, large relevant studies have been found on this topic.
RNA-seq technology allows us to observe changes in mRNA expression in OSCC cells after treatment with evodiamine, providing insights into its potential mechanisms of action. In this study, initially, the results of amino acid metabolism pathways were compared with the RNA-seq sequencing data after evodiamine treatment. Three genes were revealed to potentially serve as key targets for evodiamine in amino acid metabolism pathways. Among them, EPRS has been recently reported as a therapeutic target for malignant tumors and has been used as a target for halofuginone treatment36 and subsequent drug development.37 However, there is currently no research on EPRS in the context of OSCC or evodiamine. Subsequently, through bioinformatics analysis, EPRS expression in OSCC tissues was found higher than in normal tissues, and high EPRS expression was associated with poor survival prognosis in OSCC patients. Therefore, we hypothesized that evodiamine may potentially inhibit the development of tumors by targeting EPRS.
Through the OSCC animal model, evodiamine was discovered which leads to a decrease in EPRS expression and limits the size of tumor tissue. In OSCC cell models, evodiamine was also found to inhibit tumor cell proliferation and reduce the expression of EPRS. However, the exact mechanism by which evodiamine regulates EPRS expression remains unclear. According to this article's result, we speculate that it may be related to transcription factors and have made certain predictions through network databases. SCYL2 has been reported as the downstream protein of EPRS in 2021.20 SCYL2 (SCY1-like protein 2) interacts with EPRS to enhance the WNT/GSK-3β/β-catenin signal transduction, leading to cancer cell proliferation and tumor growth which was reported in gastric cancer. However, the effect of SCYL2 in OSCC still needs further research endeavors.
Research on specific genes plays a crucial role in the diagnosis, treatment, and prognosis of OSCC. For example, NUPR1,38 microRNA-485-5p,39 and STAT40 are known to play an essential role in OSCC development. However, the role and underlying mechanisms of EPRS in OSCC have not been thoroughly explored. Therefore, EPRS expression in Cal27 and SAS cells was knocked down to illustrate that cell proliferation and Glu-tRNA-related protein synthesis were inhibited by EPRS, which was similar to the effects observed after treatment with evodiamine. Eventually, in rescue experiments, overexpressing EPRS was identified to reverse the inhibitory EVO on cell glutamate protein synthesis and cell proliferation.
5 CONCLUSIONS
In conclusion, the research indicates that evodiamine, by downregulating EPRS expression, impacts the process of Glu-tRNA synthesis protein in OSCC cells and inhibits their proliferative activity. First, this study establishes the association between EPRS and the prognosis of OSCC, suggesting its potential as a therapeutic target for OSCC. Furthermore, this research validates the potential mechanism by which evodiamine controls OSCC proliferation, laying the foundation for future developments and studies involving evodiamine.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
ETHICS STATEMENT
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of School/Hospital of Stomatology, Lanzhou University, (Date 4 April, 2023/No.LZUKQ-2023-024).




