Salvianolic acid A improves nerve regeneration and repairs nerve defects in rats with brain injury by downregulating miR-212-3p-mediated SOX7
Abstract
This study was to probe the protective effects and mechanisms of salvianolic acid A (SAA) on cerebral ischemia–reperfusion injury (CIRI). The middle cerebral artery occlusion model (MCAO) was established in rats. Rats' behavior, neurological deficits, brain injury, inflammation, and apoptosis in the brain tissue were evaluated. The inflammatory response and apoptosis of PC12 cells induced by oxygen glucose deprivation/reperfusion (OGD/R) were detected. SAA-mediated changes in miR-212-3p, SOX7, and Wnt/β-catenin pathway were determined, and the targeting relationship between miR-212-3p and SOX7 was clarified. SAA alleviated the neurological deficits and brain injury of MCAO rats and inhibited the inflammatory response and apoptosis of OGD/R-conditioned PC-12 cells. SAA upregulated miR-212-3p, Wnt3a, and β-catenin, whereas inhibited SOX7 levels. Silencing miR-212-3p counteracted the protective effect of SAA in the context of CIRI. SOX7 was a target protein of miR-212-3p. Silencing SOX7 based on SAA and miR-212-3p knockdown suppressed OGD/R-induced inflammation and apoptosis and increased Wnt3a and β-catenin levels in PC12 cells. SAA can improve the brain and nervous system injury caused by cerebral ischemia–reperfusion by upregulating miR-212-3p, thereby inhibiting SOX7 and activating the Wnt/βcatenin signaling pathway.
1 INTRODUCTION
Cerebral ischemia–reperfusion injury (CIRI) is causal of death and disability in patients with cerebrovascular diseases, which is often related to various neurological diseases such as ischemic stroke, ischemic cerebrovascular disease, and vascular dementia.1 Clinically, there is still a lack of effective protective drugs to alleviate and control the neurological defects caused by inflammation and neuronal apoptosis.2 At present, anti-inflammatory therapy is effectuated in the early stage of CIRI,3 but relevant therapeutic drugs are limited. Water-soluble salvianolic acid A (SAA) is a pharmacological compound isolated from Salvia miltiorrhiza that improves blood circulation and maintains cardiovascular and cerebrovascular health. It has anti-inflammatory and antioxidant effects, protects the blood–brain barrier, and maintains the stable structure and number of neurons.4-7 It has been found that SSA attenuates CIRI-induced rat brain damage, inflammation, and apoptosis by regulating miR-499a/DDK1.8 However, other possible molecular mechanisms of SAA are not completely identified. Therefore, the study of the mechanism of SAA is helpful in providing new strategies and methods for treating CIRI.
microRNAs (miRNAs) are non-coding RNAs that modulate gene expression at the post-transcriptional level.9, 10 Abundantly existing in the nervous system, miRNAs take responsibility for the transport and translation of mRNA in neurons and may control cell fate.11, 12 For example, miR-146a regulates innate immune responses and inflammatory signaling in specific immune and brain cell types.13, 14 miR-132 decides the differentiation of embryonic stem cells into dopamine neurons15 and contributes to dendritic development and dendritic structure formation in adult hippocampus.16 Notably, although only miR-132 has been studied in neurons, miR-132 shares the same seed region with miR-212, and most of their targets are similar.17 miR-212 has been found to play a powerful regulatory role in the central nervous system.18 miR-212 gene locus is a key regulator of brain cognitive ability.19 Recent evidence suggests that miR-212 promotes arterial generation after lower limb ischemia by modulating the radar adsorb support-mitogen-activated protein kinase pathway.20 In addition, miR-212 promotes neuronal survival and ameliorates ischemic brain injury by regulating sirtuin 2.21 Therefore, it is assumed that miR-212 may repair brain injury by SAA and protect the nervous system.
SOX family is a typical regulator of cell fate determination during development.22, 23 As a member of the SOX family, SOX7 is detected during embryonic development, most abundant in organs, such as brain and heart.24 Current studies have confirmed that SOX7 mRNA is regulated by miRNAs25-28 and SOX7 can accelerate neuronal apoptosis by interfering with β-catenin activity.29, 30 The Wnt/β-catenin pathway is highly conserved and mainly regulates cell behavior and inter-cellular communication. The Wnt/β-catenin pathway mainly functions in regulating neuronal survival and apoptosis31 and is closely related to neuronal apoptosis after CIRI.32
This study was to explore the functions of SAA, miR-212, and SOX7 in CIRI and to verify their relationship, so as to provide possible therapeutic strategies for this disease.
2 MATERIALS AND METHODS
2.1 Chemicals and reagents
SAA (≥98%) was obtained from Winherb (Shanghai, China). TUNEL staining kit and Nissl staining kit were purchased from Beyotime (Shanghai, China). SOX7 (ab220293, Abcam), Wnt3a (ab219412, Abcam), β-catenin (ab16051, Abcam), Bax (2772, CST), Cleaved Caspase-3 (9661, CST), p65 (8242, CST), p-p65 (3033, CST), and GAPDH (ab9485, Abcam) were utilized for Western blot.
2.2 Animal modeling and treatment
This study was approved by the Affiliated Nantong Hospital of Shanghai University (The Sixth People's Hospital of Nantong), and all procedures complied with the National Institutes of Health Guide for the Use of Laboratory Animals. Forty-five 6-week-old Sprague–Dawley male rats, weighing 250–350 g, were provided by Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). The 45 rats were divided into nine cages with five rats per cage. Rats were housed at 25 ± 2°C and 50%–60% humidity, and adaptively fed for 7 days in a cycle of 12/12 h light/dark.
Surgical methods for middle cerebral artery occlusion (MCAO) were described in detail previously.33 In brief, an intraperitoneal injection of 1% pentobarbital sodium (0.06 g/kg) was administrated and each anesthetized rat was connected to a Doppler blood flow monitor detector to cerebral blood flow. A longitudinal incision (1 cm) was prepared on the neck. With the external carotid artery ligated with a 5–0 silk suture, the internal carotid artery was inserted with a 7–0 surgical nylon monofilament with a rounded end. During 120-min MCAO, the incision was treated with 0.9% sodium chloride. The monofilament was then withdrawn to allow blood flow to reperfusion, and the incision and skin were sutured. No surgical nylon monofilament was inserted in the sham group. At 24-h postoperation, an intraperitoneal injection of 1% pentobarbital sodium (0.06 g/kg) was required to anesthetize rats, among which three in each group were perfused with formalin through the heart to obtain brain tissues, three for brain edema study, and three for gene and protein expression analysis.
The injection dose and protocol of SAA were based on the previous literature.8, 34 Rats in Sham and MCAO groups were intraperitoneally injected with 1 mL/kg normal saline 15 min before operation. Rats in MCAO + SAA group were intraperitoneally injected with 20 mg/kg SAA (dissolved in normal saline; Shanghai Yuanye Bio-Technology Co., Ltd, Shanghai, China). Rats in MCAO + SAA + antagomir-NC and MCAO + SAA + antagomir-212-3p groups were intraperitoneally injected with 20 mg/kg SAA and 10 mg/kg antagomir-NC or antagomir-212-3p, respectively.
2.3 Brain edema
With the olfactory bulb and brainstem removed, the brain tissue was weighed and recorded as wet weight (W). After drying in an oven at 100°C for 48 h, the tissue was weighed and recorded as dry weight (D). The brain water content was (W − D)/W × 100%.
2.4 Neurological deficit score
Neurological deficits of rats were measured by the Zea-Longa scale: 0 point = normal behaviors; 1 point = left anterior extension disorder (mild); 2 points = turning to left while crawling (moderate); 3 points = turning to the hemiplegic side while walking (severe); and 4 point = unable to walk (unconscious).
2.5 TUNEL staining
The collected brain tissue was fixed with 4% paraformaldehyde for 48 h, embedded in paraffin, and processed with a microtome (RM2235, Leica, Germany) to produce sections (5 μm). Apoptosis was detected by the TUNEL staining kit (Beyotime, Shanghai, China). Briefly, after paraffin removal with xylene and rehydration with ethanol, the slices were permeabilized with 0.1% Triton X-100 (ST795, Beyotime). Antigen retrieval was performed using citric acid–sodium citrate solution. Next, sections were mixed with 50 μL TUNEL reaction mixture (45 μL labeling buffer and 5 μL TdT enzyme solution) for 1 h, treated with DAPI (C1002, Beyotime) to stain nuclei for 5 min, imaged with a fluorescence microscope (Olympus, Japan).
2.6 HE staining
After drying, the paraffin sections were soaked in xylene, dehydrated in gradient alcohol (100%, 90%, 80%, 70%), rinsed in distilled water, stained with hematoxylin for 7 min and with eosin for 5 s. After gradient dehydration with 80%, 95%, and absolute ethanol, the sections were soaked in xylene again and finally sealed with neutral glue. Finally, the slices were observed and photographed under an optical light microscope (BX51; Olympus Corporation) at ×400 magnification.
2.7 Nissl staining
Nissl staining was performed according to the instructions of the Nissl staining kit (Beyotime). Paraffin-embedded sections (5 μm) were dewaxed with xylene, hydrated with gradient ethanol, washed with ddH2O, stained with 0.5% methylene blue at 37°C for 10 min, and differentiated at 37°C for 10 min. Next, the sections were treated with 0.5% ammonium molybdate solution at 37°C for 5 min, washed with distilled water to prevent decolorization, dehydrated with anhydrous ethanol, and sealed with neutral glue after xylene clearance. Morphological observation of cortical neurons was conducted under a light microscope (BX51; Olympus) at × 400 magnification.
2.8 PC12 cell culture
Rat pheochromocytoma (PC12) cells were cultured in DMEM (Invitrogen) containing 10% FBS (HyClone) and 1% penicillin and/streptomycin (Invitrogen) with 95% humidity and 5% CO2 at 37°C.
2.9 Cell transfection
miR-212-3p mimic/inhibitor and corresponding negative controls (mimic-NC, inhibitor-NC), siRNA of SOX7, and the corresponding negative control (si-NC) were synthesized by Genechem (Shanghai, China) and confirmed by DNA sequence analysis. Transient transfection was done using manufacturer Lipofectamine 2000 (Invitrogen), and the medium was renewed after 6 h. The transfection efficacy was checked after 18 h by RT-qPCR.
2.10 Oxygen glucose deprivation/reperfusion model
Oxygen glucose deprivation/reperfusion (OGD/R) was carried out as previously reported.35 In brief, PC12 cells were cultured in DMEM without FBS and glucose in an incubator (5% CO2 and 95% N2). After 105 min of OGD, PC12 cells were treated with 125 μg/mL SAA, miR-212-3p mimic or miR-212-3p inhibitor, and si-SOX7. After 120 min of OGD, the cells were given normal DMEM with 10% FBS and placed in an incubator (5% CO2 and 95% O2) for 24 h. Cells in the control group were cultured with normal DMEM and 10% FBS for the same incubation times.
2.11 Flow cytometry
PC12 cells were tested for apoptosis using Annexin V-FITC/PI Apoptosis Detection Kit (Solarbio, CA1020). Apoptotic cells (Annexin V+ and PI−) and necrotic cells (Annexin V+ and PI+) were detected by flow cytometry.
2.12 RT-qPCR
Total RNA was produced by TRIzol Reagent (Invitrogen) and quantified using Nanodrop 2000. miRNA and mRNA were reverse-transcribed using Taqman® MicroRNA Reverse Transcription kit (Invitrogen) and cDNA Synthesis Kit (Thermo Fisher Scientific), respectively, which was subjected to RT-qPCR based on SYBR Premix Ex TaqTM II kit (RR820A, Takara) and data analysis using Biosystems 7900 thermocycler (Thermo Fisher Scientific). Measurements of gene expression were done using the 2−ΔΔCt method. Table 1 presents primer sequences.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| GAPDH | GTCTCCTCTGACTTCAACAGCG | ACCACCCTGTTGCTGTAGCCAA |
| U6 | CTCGCTTCGGCAGCACATATACT | ACGCTTCACGAATTTGCGTGTC |
| miR-212-3p | GGTAACAGTCTCCAGTCA | GCAATTGCACTGGATACG |
| SOX7 | CAAGGATGAGAGGAAACGTC | CTCTGCCTCATCCACATAGG |
- Abbreviations: GAPDH, glyceraldehyde-3phosphate dehydrogenase; miR-212-3p, microRNA-212-3p; SOX7, SRY-box transcription factor 7.
2.13 Western blot
Cell samples and tissue samples were lysed on ice with RIPA lysis buffer supplemented with phosphatase and protease inhibitors and centrifuged at 15,871g for 10 min at 4°C. The protein concentration was then determined using the BCA kit (Sigma-Aldrich). After separation by 10% SDS-PAGE, protein samples were transferred to PVDF membranes, blocked with 5% skim milk for 1 h, and incubated with the following diluted primary antibodies overnight at 4°C: SOX7 (ab220293, Abcam), Wnt3a (ab219412, Abcam), β-catenin (ab16051, Abcam), Bax (2772, CST), Cleaved Caspase-3 (9661, CST), p65 (8242, CST), p-p65 (3033, CST), and GAPDH (ab9485, Abcam). Next, the membranes were incubated with the corresponding horseradish peroxidase-conjugated secondary antibody (CST) for 1 h and developed using the ECL chemiluminescence kit (ultrassignal, China).
2.14 Enzyme-linked immunosorbent assay
Rat brain tissue was taken after anesthesia, and the blood stains were washed away with pre-cooled 0.9% normal saline, and the homogenate was mechanically ground in 200 mg/mL 0.9% normal saline, centrifuged at 12000 rpm at 4°C for 10 min, and the supernatant was collected. PC12 cell supernatants were collected after centrifugation at 4000 rpm for 15 min at 4°C.
IL-1β, IL-6, and TNF-α in brain tissue and PC12 cell supernatants were measured at OD 450 nm using a commercial ELISA kit (Abcam).
2.15 Luciferase activity
starBase 3.0 (http://starbase.sysu.edu.cn/) analyzed the binding sites between miR-212-3 p and SOX7. The 3'UTR sequences of wild-type and mutant SOX7 containing miR-212-3p-binding sites (WT/MUT-SOX7) were cloned into pMIR plasmids (Ambion, Shanghai, China). The luciferase reporter assay system was performed in 96-well plates, and the aforementioned luciferase reporter vector and miR-212-3p mimic or mimic NC were co-transfected into PC12 cells using Lipofectamine 3000 (Invitrogen). After 24 h, fluorescence values were quantified using a dual luciferase reporter assay kit (Promega) and analyzed using SpectraMax M5 instrument software (Molecular devices, USA).
2.16 RIP experiment
The Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, USA) was utilized to measure SOX7 and miR-212-3p enrichment. PC12 cell lysates were produced using RIP lysis buffer and mixed with Ago2- or IgG-bound magnetic beads for 6 h at 4°C. The eluted immunoprecipitates were finally conditioned to PCR analysis.
2.17 Co-immunoprecipitation
PC12 cells were cleaved using RIPA cleavage buffer (Pierce, USA). Protein A/G beads (Santa Cruz, USA) were co-incubated with primary antibody or IgG at 4°C for 6 h. The cell lysate was added to the mixture of beads and antibodies and incubated overnight at 4°C, washed with pre-cooled PBS three times (10 min each time), and analyzed by Western blot.
2.18 Data analysis
Data extracted from all experiments (at least three biological replicates) were expressed as mean ± standard deviation (SD). Statistical analysis was performed using a t-test for the comparison of two groups. A one-way analysis of variance (ANOVA) with a Bonferroni post-test was used for more than two groups. p < 0.05 was considered statistically significant.
3 RESULTS
3.1 SAA-induced protection against CIRI is related to miR-212-3p
Figure 1A shows the chemical structure of SAA. An animal model of CIRI was constructed, and Figure 1B presents the experimental design process. Then, the expression of miR-212-3p in the brain tissue of MCAO rats was detected by RT-qPCR. The results showed that miR-212-3p expression dropped in the brain tissue of MCAO-modeled rats, but could be restored after SAA treatment. However, SAA-induced increase in miR-212-3p levels was suppressed when antagomir-212-3p was injected.
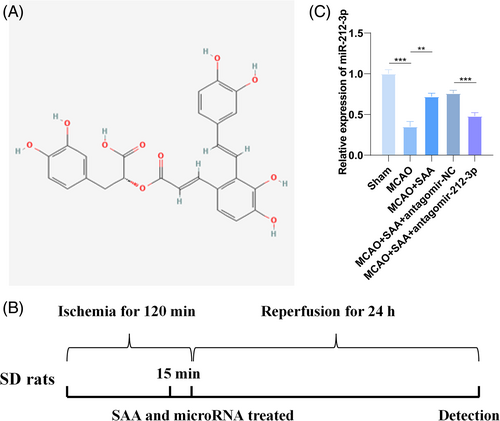
3.2 SAA alleviates brain injury, neurological deficits, and inflammatory response by upregulating miR-212-3p in MCAO rats
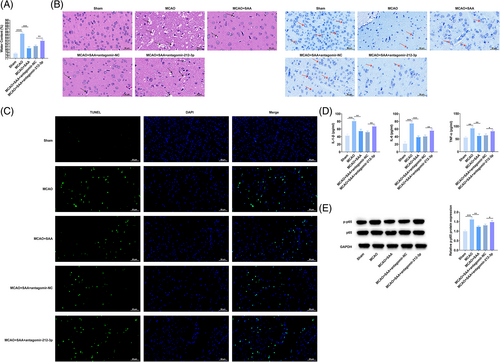
Zea Longa method was allowed to evaluate and score the neurological function of rats after CIRI. The observation results manifested that sham operation had no obvious effect on rats' behaviors and the neurological function score was 0. After MCAO modeling, rats behaved abnormally, with higher scores. SAA treatment lowered the score of MCAO rats (Table 2), indicating that SAA can improve nerve function in MCAO rats. In addition, the results of brain water content measurement determined that SAA treatment decreased brain water content (Figure 2A). HE staining and Nissl staining were used to observe the pathological conditions of the cerebral cortex of MCAO rats. It was found that the neuronal structure of MCAO rats was disordered and loose, the cell body was swollen, the number of neurons was reduced, and there were vacuoles between cells, while the neuronal structure of rats after SAA treatment was relatively intact and the number of neurons was large, and the number of vacuoles between cells was reduced (Figure 2B). TUNEL staining was used to evaluate neuronal apoptosis in the cerebral cortex of MCAO rats. It could be observed that MCAO operation increased the number of positive cells, whereas SAA attenuated this effect (Figure 2C). Additionally, IL-6, IL-1β, and TNF-α contents in the brain tissue of rats in MCAO rats were elevated, while suppressed after SAA treatment (Figure 2D). Notably, p-p65 protein expression was augmented in MCAO rats, but the opposite was observed in SAA-treated MCAO rats (Figure 2E). Meanwhile, antagomir-212-3p injection aggravated MCAO-induced damages to rat brain tissues and suppressed the ameliorative effects of SAA (Figure 2A–E). These results suggest that SAA can improve brain injury and nerve function in MCAO rats and inhibit inflammatory response by up-regulating miR-212-3p.
| Group | Neurological deficient scores (0–4) |
|---|---|
| Sham | 0.00 ± 0.00 |
| MCAO | 3.25 ± 0.56*** |
| MCAO + SAA | 1.25 ± 0.62* |
| MCAO + SAA + antagomir-NC | 1.50 ± 0.49 |
| MCAO + SAA + antagomir-212-3p | 2.50 ± 0.43* |
- * p < 0.05;
- ** p < 0.01;
- *** p < 0.001;
- **** p < 0.0001.
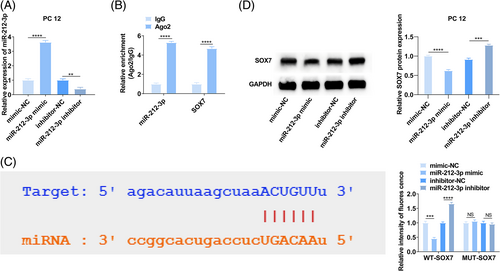
3.3 MiR-212-3p targets SOX7
The potential targets of miR-212-3p were analyzed by starBase 3.0 (http://starbase.sysu.edu.cn/), and 10 proteins with binding sites to miR-212-3p were selected. miR-212-3p mimic/inhibitor was transfected into PC12 cells, respectively, and RT-qPCR ensured the success of transfection to alter miR-212-3p expression (Figure 3A). After enhancing miR-212-3p, there were only three proteins (FXR1, SOX7, and SOD2) presented expression alternation. Next, RIP experiment confirmed that SOX7 and miR-212-3p were enriched in Ago2-coating beads relative to IgG control group. (Figure 3B), indicating that miR-212-3p can bind to SOX7. Meanwhile, the dual luciferase assay found that when co-transfected with miR-212-3p mimic and WT-SOX7, luciferase activity decreased in PC12 cells (Figure 3C). Furthermore, immunoblotting confirmed the impairing effect of miR-212-3p overexpression and the promoting effect of miR-212-3p silencing on SOX7 protein expression (Figure 3D). These results indicated that miR-212-3p targets SOX7 expression.
3.4 SAA-mediated miR-212-3p improves the inflammation and apoptosis of OGD/R-treated PC12 cells
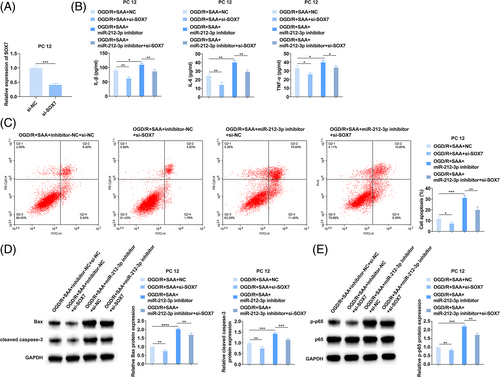
OGD experiments were performed on PC12 cells to simulate the changes in gene expression and apoptosis after cerebral ischemia. Figure 4A shows the design process of OGD/R for PC12 cells. A safe dose of 125 μg/mL SAA was selected to treat PC12 cells.34 Then, the contents of inflammatory factors (IL-6, IL-1β, TNF-α) were detected by ELISA, apoptosis of PC12 cells was detected by flow cytometry, and apoptosis- (Bax and cleaved caspase-3) and inflammation-related proteins (p65 and p-p65) were detected by Western blot. In OGD/R-stimulated PC12 cells, it could measure elevated IL-6, IL-1β, and TNF-α contents (Figure 4B), promoted apoptosis (Figure 4C), increased protein expression of Bax, cleaved caspase-3, and p-p65 (Figure 4D). After OGD/R injury, miR-212-3p levels decreased and SOX7 mRNA levels elevated in PC12 cells (Figure 4E). Functionally, for PC12 cells injured by OGD/R, SAA treatment attenuated inflammatory response and apoptosis, and also enhanced miR-212-3p and suppressed SOX7 mRNA expression (Figure 4B–E). In the meantime, these effects mediated by SAA could be reinforced by miR-212-3p mimic-induced upregulation of miR-212-3p, while suppressed by miR-212-3p inhibitor-mediated downregulation of miR-212-3p (Figure 4B–E). These results suggest that SAA can improve the inflammation and apoptosis of OGD/R-treated PC12 cells by upregulating miR-212-3p.
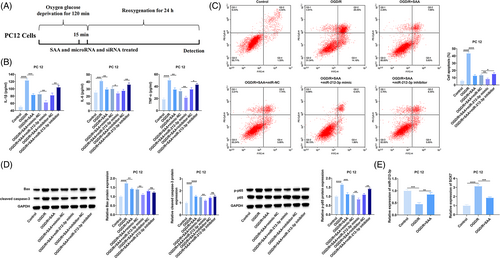
3.5 SAA inhibits the inflammatory response and apoptosis of PC12 cells by the miR-212-3p/ SOX7 axis
Rescue experiments were allowed in the OGD/R model of PC12 cells. First, si-SOX7 was transfected into PC12 cells, and SOX7 expression was reduced (Figure 5A). Subsequently, miR-212-3p inhibitor and si-SOX7 were co-transfected into SAA-treated OGD/R-injured PC12 cells. ELISA and flow cytometry noticed that silencing SOX7 blocked the pro-inflammatory response and pro-apoptotic effect of miR-212-3p inhibitor on PC12 cells (Figure 5B,C), as well as the promoting effect on Bax, cleaved caspase-3, and p-p65 protein expression (Figure 5D). These results suggest that SAA inhibits inflammatory response and apoptosis of PC12 cells through the miR-212-3p/SOX7 axis.
3.6 SAA activates Wnt/β-catenin pathway by regulating miR-212-3p/SOX7 axis
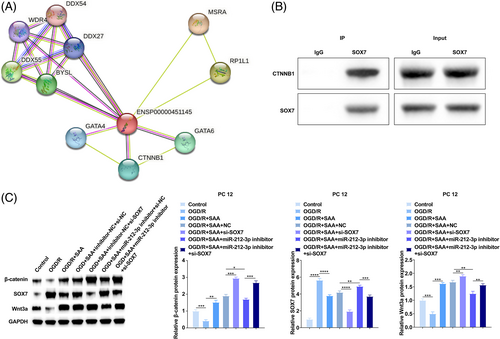
SOX7 interacting proteins were predicted in STRING database (https://cn.string-db.org/).36 SOX7 and CTNNB1 may interact with each other, with a score of about 0.7, which is highly credible (Figure 6A). The interaction between SOX7 and CTNNB1 was verified by co-immunoprecipitation (Figure 6B). CTNNB1 is a positive regulator of the Wnt/β-catenin pathway and the activation of Wnt/β-catenin pathway can ameliorate CIRI. As determined, SOX7 in OGD/R-conditioned PC12 cells was increased while Wnt3a and β-catenin levels were decreased, which was contrary to the results obtained in OGD/R-conditioned PC12 cells after treatment with SAA. Meanwhile, miR-212-3p knockdown increased SOX7 and inhibited Wnt3a and β-catenin levels, but these effects were counteracted by silencing of SOX7 (Figure 6C). In summary, SAA activates the Wnt/β-catenin pathway by regulating the miR-212-3p/SOX7 axis.
4 DISCUSSION
The brain is the most sensitive organ to hypoxia, and cerebral ischemia can lead to irreversible damage such as inflammation and apoptosis of neurons.37 SAA has a protective effect on CIRI, which can improve brain injury and brain edema, repair neurological deficits, and inhibit brain inflammatory response.38 SAA can block the NF-κB signaling pathway to inhibit inflammation and alleviate brain injury.39 In addition, SAA by activating the SIRT1/β-catenin signaling pathway can reduce alcohol-induced oxidative stress and chronic liver injury.40 Therefore, this study attempted to identify other possible molecular mechanisms of SAA in CIRI, and confirmed that SAA could inhibit SOX7 and activate the Wnt/β-catenin pathway by upregulating miR-212-3p, thereafter improve brain edema and neurological dysfunction, inhibit inflammation, and neuronal apoptosis.
miRNAs are involved in many physiological and pathological processes, such as nervous system development and inflammation. For example, miR-212-3p inhibits early neurogenesis, which is expected to become a therapeutic target for neurogenic diseases. miR-212-3p is downregulated in spinal cord injury (SCI) model, which could improve the functional recovery of SCI rats and inhibit neuronal apoptosis. This study found that miR-212-3p expression dropped during CIRI in rats, while miR-212-3p expression was restored after SAA treatment, and brain edema, brain tissue morphology, and nervous system function were improved. In addition, antagomir-212-3p suppressed the protective effect of SAA on CIRI in rats, indicating that SAA can upregulate miR-212-3p to improve brain injury and neurological defects. In conclusion, this study found for the first time that SAA improved brain injury and neural function in MCAO rats by up-regulating miR-212-3p expression. In addition, a previous study found that SSA attenuates CIRI-induced rat brain damage, inflammation and apoptosis by regulating miR-499a/DDK1,8 which is another possible mechanism by which SAA plays an improving role.
Subsequently, the study detected SOX7 upregulation in rat CIRI model and OGD/R-induced PC12 cells, and subsequently confirmed that SOX7 was a downstream target of miR-212-3p. SOX7 can act transcriptionally and is related to cell fate determination and neuronal apoptosis regulation.29, 30 Therefore, an OGD/R-induced PC12 cell model was established to simulate the cerebral ischemia environment. Based on the cell model, the study recognized and verified SAA-induced protection against OGD/R-mediated inflammation and apoptosis in PC12 cells. Furthermore, it was noted that miR-212-3p depletion weakened the protective effects of SAA on PC12 cells, and further treatment with SOX7 silencing counteracted the threatening effect of miR-212-3p depletion.
miR-376b-5p can improve ischemic brain injury by inhibiting SOX7 and activating Wnt/β-catenin pathway. This study determined that SAA treatment elevated Wnt3a and β-catenin levels, miR-212-3p knockdown reduced Wnt3a and β-catenin, while silencing SOX7 limited the effect of miR-212-3p knockdown on the Wnt/β-catenin pathway. CTNNB1 was tested to be a potential interaction protein of SOX7, and a key element of the Wnt signaling pathway. Therefore, it was hypothesized that SOX7 may inhibit the Wnt/β-catenin signaling by acting on CTNNB1 protein. In conclusion, our study confirmed that SAA can activate the Wnt/β-catenin pathway by regulating the miR-212-3p/SOX7 axis.
Limitations also are shown in this study. For example, how SAA regulates the Wnt/β-catenin signaling through SOX7 has not been thoroughly studied. Therefore, in the subsequent experiments, the molecular mechanism of Wnt/β-catenin pathway activated by SAA deserves studies in CIRI.
5 CONCLUSION
SAA can regulate the miR-212-3p/SOX7 axis to activate the Wnt/β-catenin pathway, improve brain injury, edema, and neurological deficits caused by CIRI, and provide new therapeutic methods and strategies for ischemic brain injury.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




