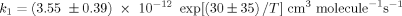The gas phase reactivity of aliphatic polyethers towards OH radicals: Measurements and predictions
Abstract
Using the flash photolysis resonance fluorescence technique, absolute rate constants were determined for the gas phase reactions of hydroxyl radicals with a series of aliphatic polyethers. At 298 K, the measured rate constants (in units of 10−12 cm3 molecule−1 s−1) were: 2,2-dimethoxypropane, (3.9 ± 0.2); 2,2-diethoxypropane, (11.7 ± 1.3); 1,2-dimethoxypropane, (14.3± 1.5); 2-methoxyethylether, (17.5 ± 1.1); 2-ethoxyethlyether, (26.8 ± 2.4); 1,1-dimethoxyethane, (8.9 ± 1.0); and 1,1,3-trimethoxypropane, (16.7 ± 1.0).
The temperature dependencies of the rate constants for 2,2-dimethoxypropane and 2,2-diethoxypropane, reactions (1) and (2), were studied over the temperature range 240–440 K and are expressed by the Arrhenius equations: