Studies on the composition and kinetics of chromium(III)-alanine system
Abstract
Kinetics of the complex formation of chromium(III) with alanine in aqueous medium has been studied at 45, 50, and 55°C, pH 3.3–4.4, and μ = 1 M (KNO3). Under pseudo first-order conditions the observed rate constant (kobs) was found to follow the rate equation:
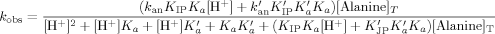
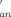 , KIP, and K
, KIP, and K ) were calculated. Activation parameters for anation rate constants, ΔH≠(kan) = 25 ± 1 kJ mol−1, ΔH≠(k
) were calculated. Activation parameters for anation rate constants, ΔH≠(kan) = 25 ± 1 kJ mol−1, ΔH≠(k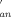 ) = 91 ± 3 kJ mol−1, and ΔS≠(kan) = −244 ± 3 JK−1 mol−1, ΔS≠(k
) = 91 ± 3 kJ mol−1, and ΔS≠(kan) = −244 ± 3 JK−1 mol−1, ΔS≠(k ) = −30 ± 10 JK−1 mol−1 are indicative of an (Ia) mechanism for kan and (Id) mechanism for k
) = −30 ± 10 JK−1 mol−1 are indicative of an (Ia) mechanism for kan and (Id) mechanism for k routes (‥substrate Cr(H2O)
routes (‥substrate Cr(H2O) is involved in the k route whereas Cr(H2O)5OH2+ is involved in k′ route). Thermodynamic parameters for ion-pair formation constants are found to be ΔH°(KIP) = 12 ± 1 kJ mol−1, ΔH°(K
is involved in the k route whereas Cr(H2O)5OH2+ is involved in k′ route). Thermodynamic parameters for ion-pair formation constants are found to be ΔH°(KIP) = 12 ± 1 kJ mol−1, ΔH°(K ) = −13 ± 3 kJ mol−1 and ΔS°(KIP) = 47 ± 2 JK−1 mol−1, and ΔS°(K
) = −13 ± 3 kJ mol−1 and ΔS°(KIP) = 47 ± 2 JK−1 mol−1, and ΔS°(K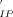 ) = 20 ± 9 JK−1 mol−1.
) = 20 ± 9 JK−1 mol−1.




