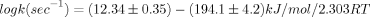Effect of substituents in the gas-phase elimination kinetics of β-substituted ethyl acetates
Abstract
The kinetics of gas-phase elimination of 3-methyl-1-butyl acetate and 3,3-dimethyl-1-butyl acetate into acetic acid and the corresponding substituted butenes have been measured over the temperature range of 360–420°C and the pressure range of 63–250 Torr. The reactions are homogeneous in both clean and seasoned vessels, obey first-order law, and are unimolecular. The temperature dependence of the rate constants is given by the Arrhenius equation
3-methyl-1-butyl acetate:
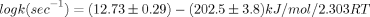
3,3-dimethyl-1-butyl acetate: