Expanding the Estimated Fetal Weight Definition of Growth Restriction by Adding Small Abdominal Circumference
Prediction of Neonatal Morbidity
The authors acknowledge the research teams at all participating clinical centers, including Christina Care Health Systems, University of California, Irvine, Long Beach Memorial Medical Center, Northwestern University, Medical University of South Carolina, Columbia University, New York Presbyterian Queens, Queens, St. Peters' University Hospital, New Brunswick, New Jersey, University of Alabama at Birmingham, Women and Infants Hospital of Rhode Island, Fountain Valley Regional Hospital and Medical Center, and Tufts University. The authors also acknowledge C-TASC and The EMMES Corporations in providing data and imaging support for this multisite study, and the assistance of GE Healthcare Women's Health Ultrasound for their support and training on the Voluson and Viewpoint products over the course of this study.
This research was supported, in part, by the Division of Population Health Research, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institutes of Health; and in part, with Federal funds for the NICHD Fetal Growth Studies and included ARRA funding (Contract Numbers: HHSN275200800013C; HHSN275200800002I; HHSN27500006; HHSN275200800003IC; HHSN275200800014C; HHSN275200800012C; HHSN275200800028C; HHSN275201000009C). KLG has contributed to this work as part of her official duties as an employee of the United States Federal Government.
This study was presented at the 44th Annual Pregnancy Meeting of the Society of Maternal-Fetal Medicine, February 10–14th, 2024, National Harbor, MD, USA.
All individuals who contributed to this work have met standard criteria for authorship. The authors report no conflicts of interest. There are no financial conflicts of interest to disclose.
Abstract
Objective
The Society for Maternal-Fetal Medicine's (SMFM) diagnostic criteria for fetal growth restriction (FGR) recently added abdominal circumference (AC) <10th percentile to estimated fetal weight (EFW) <10th percentile; however, its prediction of neonatal morbidity is unknown. Our objective was to compare the two definitions for their prediction of composite neonatal morbidity.
Methods
Secondary analysis of the Fetal Growth Study-Singletons, 2009–2013. The last ultrasound (mean 36.9 ± 2.3 weeks) was included from non-anomalous fetuses. Composite neonatal morbidity was the primary outcome: metabolic acidosis, neonatal intensive care unit stay >3 days, significant respiratory morbidities, seizures, hyperbilirubinemia requiring exchange transfusion, intrapartum aspiration, necrotizing enterocolitis, hypoglycemia, hypoxic ischemic encephalopathy, periventricular leukomalacia, sepsis, retinopathy of prematurity, or neonatal death. The secondary outcome was small for gestational age (SGA). Logistic regression modeled the association of each FGR definition with outcomes, and receiver operating characteristic area under the curve (AUC) assessed predictive ability.
Results
Of 2400 eligible individuals, 135 (5.6%) neonates had composite neonatal morbidity, and 245 (10%) were SGA. At the last ultrasound, 181 (7.5%) had FGR based on EFW alone (original definition) and 215 (9.0%) had FGR based on a small EFW or AC (expanded definition) (P < .0001). Both definitions had poor discrimination for composite neonatal morbidity (original: AUC 0.52, 95% confidence interval [CI] 0.49–0.54; expanded: AUC 0.51, 95% CI, 0.48–0.54). Both had acceptable discrimination of SGA (original: AUC 0.70, 95% CI 0.67–0.73; expanded: AUC 0.71, 95% CI 0.68–0.75).
Conclusions
Adding AC <10th percentile to the EFW <10th percentile definition of FGR significantly increased the incidence of FGR but did not improve the prediction of neonatal morbidity in a low-risk population. The SMFM guideline for FGR should be adopted with caution.
Abbreviations
-
- AC
-
- abdominal circumference
-
- AUC
-
- area under the curve
-
- BMI
-
- body mass index
-
- CI
-
- confidence interval
-
- EFW
-
- estimated fetal weight
-
- FGR
-
- fetal growth restriction
-
- NICHD
-
- National Institute of Child Health and Human Development
-
- SGA
-
- small for gestational age
-
- SMFM
-
- Society for Maternal-Fetal Medicine
Small for gestational age (SGA), defined as birthweight less than the 10th percentile, is associated with higher risk of both short- and long-term morbidity and mortality.1-7 Antenatally, fetal growth restriction (FGR) is associated with higher risk of preterm birth as well as perinatal morbidity and mortality. Increased surveillance of fetuses diagnosed with FGR is recommended with the goal to reduce intrauterine fetal death. However, diagnosing FGR remains a challenge in clinical practice.8 Traditionally, the American College of Obstetricians and Gynecologists (ACOG) and Society for Maternal-Fetal Medicine (SMFM) defined FGR as estimated fetal weight (EFW) less than the 10th percentile for gestational age, with the recognition that it was a proxy for SGA and may misdiagnose fetuses who fail to meet their growth potential. In October 2020, SMFM published a consult series that redefined FGR from an estimated fetal weight (EFW) <10th percentile to an EFW <10th percentile or an abdominal circumference (AC) <10th percentile.9 This change was primarily based on reports demonstrating that expansion of the sonographic diagnosis of FGR in utero detects a higher percentage of cases of SGA at birth.10-12 However, it is unclear whether this increased antenatal detection improves the prediction of neonates who experience neonatal morbidity. Given the increased need for fetal surveillance and potential for iatrogenic preterm birth with its associated neonatal morbidity, it is imperative to investigate whether the addition of small fetal AC as a diagnostic criterion for FGR is associated with improved detection of neonatal morbidity, and not just its SGA proxy.13, 14 Therefore, our primary aim was to compare the performance of two definitions of FGR: EFW <10th percentile alone versus the expanded definition that includes either EFW or AC <10th percentile for the prediction of composite neonatal morbidity and secondarily, SGA.
Materials and Methods
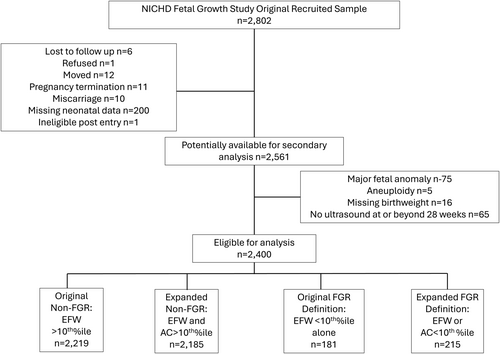
This was a secondary analysis of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies-Singletons. The NICHD Fetal Growth Study was performed to establish standards for normal fetal growth and size-for-gestational age in a multi-racial/ethnic US population. Details of this study have been previously reported.15 In short, this was a prospective cohort study of low-risk individuals (2334 without obesity [body mass index (BMI) < 30] and 468 with obesity [BMI ≥30]) recruited between 8- and 13-weeks' gestation at 12 clinical sites from July 2009 and January 2013. Recruitment was stratified by four self-identified multiracial groups (White, African American, Hispanic, Asian). Individuals were eligible for inclusion if they were between the ages of 18 and 40, had a viable singleton gestation at the dating ultrasound with no suspected structural or chromosomal anomaly, and were expected to deliver at a participating hospital. Individuals were excluded if they had any of the following medical conditions: autoimmune disorder, cancer, chronic hypertension requiring two or more medications, HIV, renal disease, diabetes mellitus, or psychiatric disorders requiring medication or any significant past pregnancy complication. In addition, individuals without obesity were excluded if they had any hematologic disorder, asthma, thyroid disorder, epilepsy or seizure disorder requiring medication. Individuals in both cohorts were also excluded if they reported smoking tobacco in the past 6 months, used illicit drugs in the past year or consumed more than one alcoholic drink per day at the time of enrollment. A full list of inclusion and exclusion criteria may be found in the original trial protocol.15 Research quality ultrasounds were performed at up to six visits where fetal biparietal diameter, head circumference, AC, and femur length were measured following a standardized protocol. Rigorous quality control procedures for training and credentialing of sonographers, coupled with quality assurance oversight, ensured that measurements acquired were accurate and reliable.16 Individuals and their providers were blinded to the results of the research ultrasounds. However, an EFW below the 10th percentile detected on a study sonogram using Bukowski's method was considered a disclosable condition and the primary provider was notified.17 Subsequent management, including obtaining a clinical ultrasound using local fetal growth standards, was at the provider's discretion. IRB approval was obtained at all participating centers, data coordinating centers, and the NICHD, and participants gave informed consent prior to data collection.
We included all individuals in the primary study with documented neonatal outcomes and at least one protocol sonogram performed at ≥28 weeks. Those with missing ultrasound or neonatal data were excluded. The final protocol ultrasound performed in the third trimester was used for determination of EFW and AC percentiles for the categorization of FGR by either of the two diagnostic definitions. The EFW was calculated using a previously reported, 4-parameter Hadlock equation.18
Our two comparative exposures of interest were the original versus the expanded definition of FGR endorsed by SMFM: EFW <10th percentile (original definition) versus EFW or AC <10th percentile (expanded definition); thus, non-FGR fetuses were defined as either having an EFW ≥10th percentile or having both EFW and AC ≥10th percentile. AC and EFW percentiles by gestational age were derived from charts published by Grantz and colleagues.19 We estimated the ability of these FGR definitions to predict composite neonatal morbidity and, secondarily, SGA at birth; SGA at birth was determined by using Fenton, sex-specific charts.20 Neonatal morbidities included in the composite outcome were selected based on expert consultation and were defined specifically to SGA based on the likelihood of the relevant outcome and included: metabolic acidosis (pH <7.1 and base deficit >12 mmol/L), neonatal intensive care unit stay greater than 3 days, pneumonia, respiratory distress syndrome, persistent pulmonary hypertension, seizures, hyperbilirubinemia requiring exchange transfusion, intrapartum aspiration (meconium, amniotic fluid, blood), mechanical ventilation at term, necrotizing enterocolitis, hypoglycemia, hypoxic ischemic encephalopathy, periventricular leukomalacia, sepsis based on blood culture, bronchopulmonary dysplasia/chronic lung disease, retinopathy of prematurity, and neonatal death.21-24 Cases were included in the composite outcome if an infant had one or more of any of the neonatal morbidities listed.
Rates of FGR using both definitions were compared using McNemar's test. Chi-square test was used to compare rates of composite neonatal morbidity and SGA by FGR definition. Test characteristics were calculated for each FGR definition, EFW <3rd percentile, and AC <10th percentile, including true positives, false positives, true negatives, and false negatives, sensitivity, specificity, positive predictive value, and negative predictive value for SGA at birth and neonatal composite morbidity. Logistic regression modeled the relationship between each FGR definition and its associated composite morbidity and SGA outcomes. These models were used to create receiver operator characteristic (ROC) curves, and the areas under the respective curves (AUC) were used to assess the screening performance of each diagnostic method for the prediction of both composite neonatal morbidity and SGA. An AUC of 0.5 was considered no discrimination, >0.5–0.7 poor discrimination, 0.7–0.8 acceptable discrimination, and >0.8 was considered excellent discrimination.25 ROC curves were also used to demonstrate the discrimination of SGA through the whole range of EFW and AC percentiles. An alpha value of 0.05 was chosen to represent statistical significance. All statistical computations were performed using SAS (SAS Institute, Cary, NC, USA) version 9.4.
Results
Out of the 2802 individuals in the NICHD Fetal Growth Study (including 8661 protocol scans), 2400 were eligible to be included in this analysis (Figure 1) and all of these were live births. Of the 2400 eligible, 181 (7.5%) were diagnosed with FGR by the original definition, with an additional 34 (total 215, 9.0%) diagnosed with FGR by the expanded definition (P < .001), a 19% relative increase. Selected characteristics of the total and both FGR populations are displayed in Table 1. The mean gestational age at the ultrasound included in the analysis was 36.9 ± 2.3 weeks. The mean gestational age at delivery in the cohort was 39.3 ± 1.4 weeks. At birth, 135 infants (5.6%) experienced composite neonatal morbidity, and 245 infants (10%) were classified as SGA.
| Characteristic | Total Population, n = 2400 | FGR Original Definition, n = 181 | FGR Expanded Definition, n = 215 |
|---|---|---|---|
| Race/ethnicity (%) | |||
| Asian | 383 (16) | 25 (14) | 28 (13) |
| Black/non-Hispanic | 645 (27) | 71 (32) | 89 (41) |
| Hispanic | 692 (29) | 59 (39) | 63 (29) |
| White/non-Hispanic | 680 (28) | 26 (14) | 35 (16) |
| Maternal age (years) | 28.2 ± 5.5 | 25.8 ± 5.1 | 26.2 ± 5.4 |
| Body mass index, kg/m2 (%) | |||
| 19–29.9 | 1995 (83) | 151 (83) | 178 (83) |
| >30.0 kg/m2 | 405 (17) | 30 (17) | 37 (17) |
| Nulliparous (%) | 1127 (47) | 98 (54) | 115 (54) |
| Married (%) | 1795 (75) | 112 (62) | 137 (64) |
| Health insurance (%) | |||
| Private or managed care | 1527 (64) | 90 (50) | 105 (49) |
| Medicaid or other | 873 (36) | 91 (50) | 110 (51) |
| Gestational age at final ultrasound, (weeks) | 36.9 ± 2.3 | 36.5 ± 2.6 | 36.7 ± 2.5 |
| Delivery gestational age (weeks) | 39.3 ± 1.4 | 39.1 ± 1.6 | 39.1 ± 1.6 |
| Delivery at <34 weeks (%) | 11 (0.5) | 2 (1.1) | 3 (1.4) |
| Delivery at <37 weeks (%) | 85 (3.5) | 10 (5.5) | 11 (5.1) |
- Data presented as n (%) or mean ± one standard deviation. FGR, fetal growth restriction.
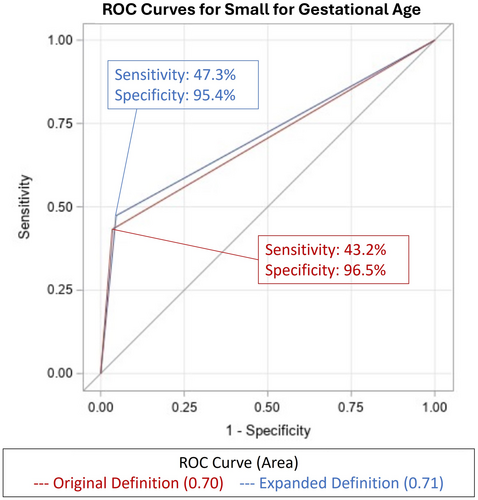
Test characteristics of each definition for the prediction of composite neonatal morbidity and SGA are found in Table 2. The sensitivity to detect composite neonatal morbidity was 10.4% with the original definition and 11.1% with the expanded definition. The sensitivity to detect SGA at birth went from 43.2% with the original definition to 47.3% with the expanded definition. Test characteristics for EFW <3rd percentile and AC <10th percentile alone are also presented for added comparison.
| Composite Neonatal Morbidity | ||||||||
|---|---|---|---|---|---|---|---|---|
| Definition | True Positive n | False Positive n | True Negative n | False Negative n | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value |
| Original | 14 | 167 | 2098 | 121 | 10% | 93% | 7.7% | 95% |
| Expanded | 15 | 200 | 2065 | 120 | 11% | 91% | 7.0% | 95% |
| EFW <3rd percentile | 8 | 50 | 2215 | 127 | 5.9% | 98% | 14% | 95% |
| AC <10th percentile | 13 | 145 | 2120 | 122 | 9.6% | 94% | 8.2% | 94% |
| Small-for-Gestational-Age | ||||||||
|---|---|---|---|---|---|---|---|---|
| True Positive n | False Positive n | True Negative n | False Negative n | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | |
| Original | 106 | 75 | 2080 | 139 | 43% | 97% | 59% | 94% |
| Expanded | 116 | 99 | 2056 | 129 | 47% | 95% | 54% | 94% |
| EFW <3rd percentile | 49 | 9 | 2146 | 196 | 20% | 99% | 85% | 92% |
| AC <10th percentile | 93 | 65 | 2090 | 152 | 38% | 97% | 38% | 97% |
- FGR, fetal growth restriction; EFW, estimated fetal weight; AC, abdominal circumference.
The risk of composite neonatal morbidity compared to non-FGR fetuses was not significantly different with either FGR definition (original definition: 7.7% versus 5.5%; P = .20; expanded definition: 7.0% versus 5.5%; P = .37; FGR versus non-FGR, respectively) (Table 3). Fetuses classified as having FGR by either definition were more likely to have SGA at birth than those not classified as FGR. Of the 181 cases identified as having FGR by the original definition, 106 (5.9%) had SGA at birth compared to 6.2% with EFW > 10th percentile (P < .001); and of the 215 cases identified by the expanded definition, 116 (54%) had SGA at birth compared to 5.9% with EFW >10th percentile or AC >10th percentile (P < .001) (Table 3).
| Outcome | EFW ≥10th Percentile (n = 2219), n (%) | Original Definition: EFW <10th percentile Alone (n = 181), n (%) | P | EFW ≥10th Percentile and AC ≥10th Percentile (n = 2185), n (%) | Expanded Definition: EFW or AC <10th Percentile (n = 215), n (%) | P |
|---|---|---|---|---|---|---|
| Composite NN morbidity | 121 (5.5) | 14 (7.7) | .20 | 120 (5.5) | 15 (7) | .37 |
| Metabolic acidosis | 3 (0.14) | 0 (0) | 3 (0.14) | 0 (0) | ||
| NICU stay >3 days | 90 (4.0) | 10 (5.5) | 89 (4.1) | 11 (5.1) | ||
| Pneumonia | 4 (0.18) | 0 (0) | 4 (0.18) | 0 (0) | ||
| Respiratory distress syndrome | 26 (1.2) | 3 (1.6) | 26 (1.2) | 3 (1.4) | ||
| Ventilator requirement | 3 (0.14) | 0 (0) | 3 (0.14) | 0 (0) | ||
| Persistent pulmonary hypertension | 1 (0.05) | 0 (0) | 1 (0.05) | 0 (0) | ||
| Seizures | 1 (0.05) | 0 (0) | 1 (0.14) | 0 (0) | ||
| Hyperbilirubinemia requiring exchange transfusion | 6 (0.27) | 1 (0.55) | 6 (0.27) | 1 (0.05) | ||
| Hypoglycemia | 1 (0.05) | 2 (1.1) | 1 (0.05) | 2 (0.93) | ||
| Necrotizing enterocolitis | 6 (0.27) | 0 (0) | 6 (0.27) | 0 (0) | ||
| Intrapartum aspiration | 7 (0.32) | 0 (0) | 7 (0.32) | 0 (0) | ||
| Hypoxic ischemic encephalopathy | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Periventricular leukomalacia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Sepsis based on blood culture | 4 (0.18) | 0 (0) | 4 (0.18) | 0 (0) | ||
| Bronchopulmonary dysplasia | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Retinopathy of prematurity | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Neonatal death | 1 (0.05) | 0 (0) | 1 (0.05) | 0 (0) | ||
| SGA | 139 (6.2) | 106 (59) | <.0001 | 129 (5.9) | 116 (54) | <.0001 |
- The table components do not sum to 100% morbidity because some fetuses had more than one morbidity component. FGR, fetal growth restriction; SGA, small for gestational age; EFW, estimated fetal weight; AC, fetal abdominal circumference; NN, neonatal; NICU, neonatal intensive care unit.
With regard to predictive ability, both FGR definitions had similar discrimination for composite neonatal morbidity. The original definition yielded an AUC of 0.52 (95% CI 0.49–0.54) and the expanded definition yielded an AUC of 0.51 (95% CI 0.48–0.54; P = .36), both demonstrating poor discrimination (Table 4). The ROC of each definition and composite neonatal morbidity is plotted in Figure 2. Similar analyses were performed for EFW <3rd percentile and AC <10th percentile alone, both yielding similarly poor discrimination for composite neonatal morbidity at an AUC of 0.52 (P = .70).
| Outcome | Original Definition: EFW <10th Percentile Alone AUC (95% CI) | Expanded Definition: EFW or AC <10th Percentile AUC (95% CI) | P |
|---|---|---|---|
| Composite neonatal Morbidity | 0.52 (0.49–0.54) | 0.51 (0.48–0.54) | .36 |
| SGA | 0.70 (0.67–0.73) | 0.71 (0.68–0.75) | .02 |
- FGR, fetal growth restriction; EFW, estimated fetal weight; AC, fetal abdominal circumference; AUC, area under the curve.

To predict SGA at birth, the original definition yielded an AUC of 0.70 (95% CI 0.67–0.73) and the expanded definition yielded an AUC of 0.71 (95% CI 0.68–0.75; P = .02) (Table 4). This result represents acceptable discrimination of SGA at birth by either definition.26 The ROC of each definition and SGA are plotted in Figure 3. Additional analyses were performed for EFW <3rd percentile and AC <10th percentile alone, yielding an AUC of 0.60 and 0.68, respectively.
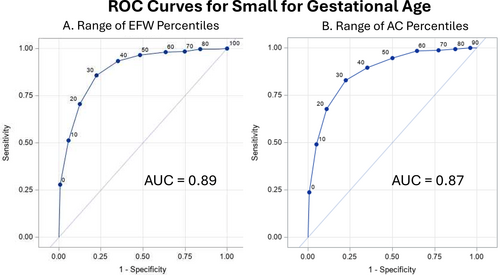
Further analyses were performed to examine the performance of the original definition as a predictor of SGA through the whole range of EFW percentiles versus the whole range of AC percentiles using an ROC curve (Figure 4, A and B). The entire range of EFW and AC percentiles was plotted yielding AUCs of 0.89 and 0.87, respectively (P = .003), representing excellent discrimination of SGA. Additionally, we examined composite neonatal morbidity through the whole range of EFW percentiles, which yielded poor discrimination at an AUC of 0.51.
Discussion
The addition of AC <10th percentile to the definition of FGR led to a 19% relative increase in the diagnosis of FGR in this population. This expanded definition had a marginally higher sensitivity and a marginally lower specificity than the original definition for both composite neonatal morbidity and SGA at birth. However, there was no difference in predictive ability for detecting composite neonatal morbidity in this low-risk population. Taken together, these findings suggest that the addition of AC <10th percentile will lead to more fetuses diagnosed with FGR, but without necessarily detecting more neonates with subsequent neonatal morbidity or SGA at birth.
Previous studies have demonstrated that a measurement of AC alone performs similarly to estimated fetal weight in the prediction of SGA at birth.10, 11 Caradeux and colleagues demonstrated in a meta-analysis that at a fixed false positive rate of 10%, AC <10th percentile had higher sensitivity for diagnosis of SGA at birth compared to EFW <10th percentile (78% versus 54%, respectively).12 Our study revealed similar discrimination of SGA for AC <10th percentile alone (AUC = 0.68) and EFW <10th percentile alone (AUC 0.71). Considering the addition of AC <10th percentile to the EFW definition of FGR, Blue and colleagues concluded that adding AC <10th percentile to the EFW definition only marginally improved the identification of SGA at birth (expanded definition: AUC 0.78, 95% CI 0.76–0.80 versus original definition: AUC 0.76, 95% CI 0.74–0.78, P = .01).27 Congruent with these findings, we found that each definition had acceptable discrimination of SGA (AUC >0.70); and although statistically different (P = .02), the AUCs were clinically similar between definitions (original definition AUC = 0.70 versus expanded definition AUC = 0.71).
While the prediction of SGA is important, missing from prior studies are robust data on whether adding small AC to the definition of FGR is a better predictor of poor neonatal outcomes. A recent single-center study revealed that broadening the diagnosis of FGR to include isolated AC <10th percentile did not significantly increase detection of fetuses at risk for composite neonatal morbidity.13 This study was limited in statistical power given low rates of FGR and neonatal morbidity—only 1.8% of their population was diagnosed with FGR and only 2.2% had neonatal morbidity. Our study adds to this existing literature, demonstrating that neither definition of FGR had satisfactory predictive ability for adverse neonatal outcomes but increased the frequency of FGR with potential risks of iatrogenic preterm delivery, unnecessary patient-related stress and anxiety, and increased resource utilization, topics for future study.
The goal of classifying fetuses as FGR is to identify fetuses that are at risk of poor outcomes, most notably fetal demise. Once FGR is diagnosed, there is an increase in antenatal testing and serial ultrasound surveillance, with potential for abnormal findings to prompt preterm delivery.8 With any testing, there is a risk of a false positive result, which in this case could lead to an iatrogenic preterm or early term delivery of a fetus that was unnecessary. Sovio et al demonstrated in a 2015 prospective cohort study that universal third trimester ultrasound screening for FGR substantially increased the number of false positive results and noted that increased identification of true positives that would be missed by selective screening was at risk for unnecessary intervention.14 Combs et al found a significant increase in the diagnosis of FGR with the expanded definition (original 9.2% versus expanded 11.6%, P < .001); thus, more screened fetuses are diagnosed using the expanded definition and are at risk of intervention.28 It is well known that iatrogenic early delivery is associated with increased neonatal morbidity.29 Similarly, our results indicate that expanding the definition of FGR to include isolated AC <10th percentile would increase FGR cases and the need for antenatal testing, thus potentially increasing unnecessary iatrogenic delivery for false-positive results and interventions for babies that may just be constitutionally small or not SGA at all, and therefore, likely not at risk of significant morbidity. Prior to adopting this expanded definition of FGR, further studies are needed to demonstrate that an expanded definition of FGR improves the prediction of clinically meaningful outcomes.
Our study found that both definitions of FGR had poor discrimination for composite neonatal morbidity. The 10th percentile cut off point has previously been shown to be a poor predictor of adverse neonatal outcomes; like our findings, Liauw et al found poor sensitivity (11–13%) and poor discrimination (AUC 0.54) of neonatal morbidity when comparing the 10th percentile cut point of three fetal growth curves (Hadlock, INTERGROWTH-21st, and World Health Organization).30 They suggested an ideal EFW cut point of the 30th–40th percentile to predict neonatal morbidity; however, using this cut point clinically would have significant cost and health care utilization implications in addition to the expanded risk of iatrogenic preterm birth as discussed above. Additional research is needed to test alternative definitions of FGR beyond a small EFW, such as the addition of abnormal Doppler findings or crossing EFW quartiles, and whether their use might be associated with measurably improved neonatal outcomes. Neonatal outcome and perinatal morbidity should be the best indicator of a clinically useful definition rather than just the finding of small birthweight-for-gestational age percentile that includes those infants who are only constitutionally small, but not at risk of significant morbidity.
Our study has limitations. The low-risk patient population may not be representative of the typical US population, making our results not applicable to all groups; however, if the definition of FGR is to be universally used, the definition needs to work well in populations at all risk levels and proven to not increase harm in those at lowest risk. Given that we utilized a sample of convenience and did not perform a priori estimates of beta error, we may have had limited power to detect differences, as composite neonatal morbidity was uncommon (5.6%) in our population. The diagnosis of FGR could be made by managing physicians independent of the research ultrasound studies and may have prompted fetal testing and altered delivery planning. However, while antenatal fetal testing protocols may prevent fetal death, its role in improving other aspects of the neonatal condition is unproven, and the use of these interventions is unlikely to affect SGA at birth.31 Additionally, our study focused on the last ultrasound prior to delivery and did not examine differences in gestational age at FGR diagnosis or trends in fetal growth.
Nevertheless, our study has several strengths. All data were collected under a well-defined study protocol, and research quality ultrasounds were performed at specified intervals, providing high-quality measurements for comparison. The strict inclusion criteria also limit potential confounders. The cohort was a racially, ethnically, and geographically diverse patient population from multiple centers across the US. Additionally, the average gestational age at the included ultrasound in our study was 36 weeks, which has been identified as having better accuracy than earlier third-trimester ultrasonography in the detection of SGA.32
The clinical utility of the addition of AC <10th percentile to the diagnostic criteria for FGR remains uncertain. Although the addition of isolated AC <10th percentile may marginally improve the prediction of SGA at birth, it does not appear to improve detection of fetuses at risk of clinically important adverse neonatal outcomes. Our results should prompt caution for the adoption of the current SMFM guidelines, especially in low-risk populations, and encourage further exploration into alternative definitions of FGR that may better identify neonates at risk of poor outcomes while not exposing pregnancies to unnecessary surveillance and the potential for iatrogenic preterm birth.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




