Pilot Study to Evaluate the Association Between Superb Microvascular Imaging (SMI) and Histologic Markers of Angiogenesis in Patients With Invasive Ductal Carcinoma
We would like to thank Dr. Sennur Ilvan and Dr. Mehmet Velidedeoglu for their valuable supervision and support. We also thank Dr. Iclal Nur Bulut for her contributions during data collation. This prospective study was funded by Scientific Research Coordination Unit of Istanbul University-Cerrahpasa (project number TSA-2021-35724). The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. [Correction added on 7 April 2025, after first online publication: An acknowledgment statement has been added.]
Abstract
Objectives
Increasing microvessel density and angiogenesis are linked to a poor prognosis in patients with invasive ductal carcinoma (IDC) of the breast. This study aims to investigate intratumoral and peritumoral microvascular flow using superb microvascular imaging (SMI) in patients with IDC and explore its association with histologic markers of tumoral angiogenesis.
Methods
Fifty-four female patients with IDC (mean age 49.5 ± 14.8 years) were evaluated using SMI before biopsy. The quantitative and qualitative vascular parameters on SMI (Adler's classification, vascular index, morphology, distribution, and penetration) were assessed. Histologic markers of angiogenesis (VEGF, ERG, and CD34) were analyzed via immunohistochemical staining in both intratumoral and peritumoral compartments of biopsy specimens. The expression levels were categorized semi-quantitatively as low or high groups based on the Allred scoring system. The association between histological and SMI parameters was analyzed. Subgroup analysis was performed according to lesion size, axillary lymph node metastasis, and histological grade.
Results
IDCs with higher expression of VEGF in the peritumoral region showed a higher vascular index (7 ± 6.4 [95% CI 5.2–8.8] versus 3.7 ± 0.9 [95% CI 2.3–5.2], P = .003) on SMI. Likewise, high peritumoral ERG expression was linked to a higher vascular index (7.2 ± 6.3 [95% CI 5.4–9.0] versus 2.4 ± 1 [95% CI 1.1–3.8], P < .001), complex vessel morphology (66.7% versus 20%, P = .024), penetrating vessels (63% versus 20%, P = .037), and central vascularity (77.6% versus 20%, P = .006). Tumors with higher intratumoral ERG expression demonstrated a more complex vessel morphology on SMI (85.7% versus 60%, P = .047). The presence of axillary lymph node metastasis was associated with a higher vascular index (10 ± 7.6 [95%CI 6.7–13.2] versus 4.2 ± 3 [95%CI 3.1–5.3], < .001), complex morphology (83.3% versus 53.3%, P = .020), and penetrating vessels (63.2% versus 50%, P = .027) on SMI, as well as higher peritumoral ERG expression (100% versus 83.3%, P = .045).
Conclusions
In this pilot study, tumors with higher neo-angiogenic activity based on histological markers correlate with increased vascular index, complex vessel morphology, penetrating vessels, and central vascularity on SMI. Larger studies are needed to assess the diagnostic accuracy and utility of risk stratification of patients.
Abbreviations
-
- ALNM
-
- axillary lymph node metastasis
-
- BI-RADS
-
- breast imaging-reporting and data system
-
- CI
-
- confidence interval
-
- ERG
-
- ETS-related gene
-
- IDC
-
- invasive ductal carcinoma
-
- MVD
-
- microvessel density
-
- SMI
-
- superb microvascular imaging
-
- VEGF
-
- vascular endothelial growth factor
Invasive ductal carcinoma (IDC) is the most common histological subtype of breast cancer, accounting for nearly 80% of all breast cancer cases.1 Despite advancements in early detection and treatment, IDC remains associated with a variable prognosis, largely dependent on factors such as tumor size, histological grade, axillary lymph node involvement, and molecular markers.2, 3 Among these, angiogenesis, the new blood vessel formation process, plays a critical role in tumor growth, metastasis, and overall prognosis.4 An increase in microvessel density (MVD), a surrogate measure of tumoral angiogenesis, is associated with more aggressive tumor behavior and a poorer clinical outcome.5
Various histologic markers of angiogenesis, such as vascular endothelial growth factor (VEGF), ETS-related gene (ERG), and CD34, have been widely studied to assess the degree of angiogenesis within the tumor microenvironment.6-8 These markers, typically detected through immunohistochemical (IHC) staining, provide important insights into the angiogenic activity of tumors, and their expression levels are valuable as prognostic tools.9, 10 However, traditional histologic methods are limited by their invasive nature, requiring tissue biopsies, and may not fully capture the dynamic vascular architecture in vivo. Additionally, tumoral heterogeneity, partial sampling from the tumor, and shrinkage in the material may lead to misinterpretations.
Recent advancements in imaging technology, particularly the development of superb microvascular imaging (SMI), offer a promising non-invasive approach to evaluating tumor vascularity in real time. SMI is an advanced Doppler ultrasound technique that utilizes clutter suppression algorithms to enable the visualization of fine microvascular flow with high sensitivity without the need for contrast agents, making it particularly suited for assessing the microvascular patterns of breast tumors.11 Several previous studies have reported that microflow assessment using SMI effectively distinguishes malignant breast masses from benign ones, and tumors with vascular parameters associated with malignancy on SMI tend to have higher histologic MVD.12 However, the relationship between SMI parameters and various histological markers of angiogenesis in distinct compartments of the tumor microenvironment remains underexplored.
This study aims to investigate the association of SMI-based microvascular parameters with 3 different histologic markers of tumoral angiogenesis (VEGF, ERG, and CD34) in patients with IDC. By evaluating intratumoral and peritumoral vascular characteristics, we seek to enhance our understanding of how in vivo imaging can reflect the underlying angiogenic processes in the tumor milieu, potentially offering a noninvasive tool for prognostic assessment in breast cancer.
Materials and Methods
Patient Selection
This prospective study was approved by the Institutional Review Board of our faculty, and informed consent was obtained from all participants. Consecutive female patients who were detected to have newly diagnosed BI-RADS 4–5 masses and referred to us for a core needle biopsy between December 2022 and February 2024 were evaluated. Cases with 1) a history of previous breast cancer surgery, chemotherapy, or radiotherapy, 2) those who were pregnant or lactating, 3) patients with complex cystic or calcified masses, and 4) patients with histopathological diagnoses other than IDC after biopsy were excluded. As a result, 54 patients who were diagnosed with IDC via core needle biopsy were included in the study.
B-Mode Ultrasonography Protocol
Ultrasonography was performed using a Toshiba Aplio α (Canon Medical Systems Inc., Tokyo, Japan) US machine and a breast-dedicated linear transducer between the 12- and 16-MHz range. The single most suspicious mass was evaluated in patients with multiple lesions. The largest diameter of the lesions in 2 orthogonal planes was measured in grayscale US. Sonographic characteristics of masses according to the 5th edition of ACR BI-RADS (shape [round-oval/irregular], margin features [circumscribed/not circumscribed], echo pattern [hypoechoic/iso-hyperechoic], posterior acoustic shadow [present/absent], and orientation [parallel/antiparallel]) were recorded.13
Superb Microvascular Imaging Protocol
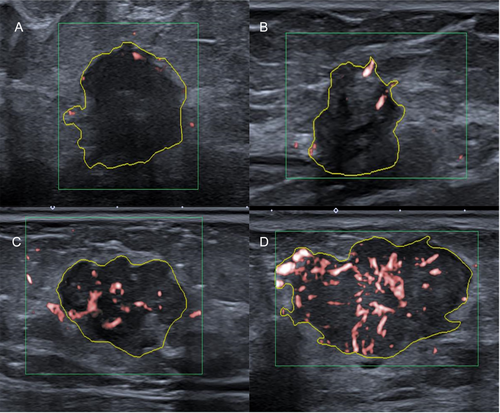
After a target mass was characterized on B-mode US, color-mode SMI was performed before the core needle biopsy. The SMI characteristics of each lesion were determined by the consensus of the 2 board-certified breast radiologists (Y.K. and S.A.K.). SMI parameters were set to velocity scale, 1.5–2.5 cm/s; mechanical index, 1.5; wall filter, 50–100 Hz; and frame rate, >50 Hz, respectively. The examination was performed without compression, and plenty of gel pads were applied over the examination area to avoid the collapse of microvessels. The plane with the most intensely vascularized site of the lesion was selected as the representative image for evaluation. Free-hand regions of interest (ROI) delineating margins of the lesions were drawn manually. We evaluated the vascular index as a quantitative parameter, along with vessel morphology, distribution, and the presence of penetrating vessels as qualitative parameters. The vascular index indicates the degree of vascularization by calculating the ratio between the Doppler signal pixel within the boundary of the lesion to the pixels of the whole lesion (Figure 1). Vessel morphology was categorized as simple (dot-like or linear) or complex (branching or shunting), while vessel distribution was categorized as peripheral (all vessels located at the margin) or central (any vessel detected within the lesion) (Figure 2). In addition, the vascularity of the lesions was evaluated semi-quantitatively based on Adler's classification (0, no flow; 1, minimal; 2, moderate; 3, significant).14 For ease of statistical evaluation, those with Adler 0–1 were classified as low-vascularized masses, and those with Adler 2–3 as highly vascularized masses.
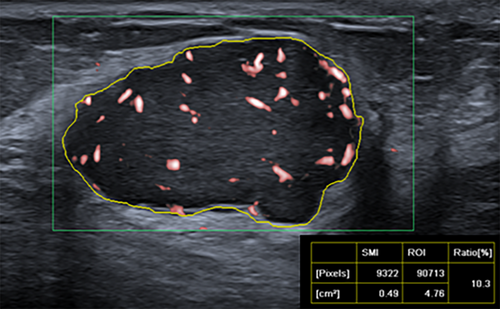
Histopathological Evaluation
Following the radiological evaluation, multiple (4–7 times) ultrasound-guided sampling was performed both from the center of the lesion and peritumoral tissue adjacent (<2 cm) to the lesion using a 14G automatic tru-cut biopsy needle. If there was a suspected axillary lymph node, a fine-needle aspiration biopsy (FNAB) was performed from this particular lymph node in the same session, accompanied by a cytopathologist. In patients without metastases, sentinel lymph node sampling was performed during surgery to determine axillary lymph node metastasis (ALNM) status. The biopsy samples were fixed in formalin and evaluated by a pathologist (T.O.) with 17 years of experience in breast pathology to determine tumor type, histological grade (based on the Elston-Ellis modification of Scarff-Bloom-Richardson grading system), hormone receptors (estrogen receptor [ER], progesterone receptor [PR]), Her-2 receptor positivity (fluorescence in situ hybridization was also performed if necessary), Ki-67 index, and the expression levels of histologic markers of angiogenesis (VEGF, ERG, and CD34). IHC staining procedures were performed using an automatic IHC staining machine (Ventana Benchmark XT, Ventana Medical Systems, Tuscon, AZ, USA). The monoclonal antibodies for ER (clone SP1; Thermo-Fisher Scientific), PR (clone 16; Novocastra), CerbB2 (HER2) (clone SP3; Thermo-Fisher Scientific), Ki-67 protein (clone 30–9; Roche), CD34 (clone QBEnd-10; Dako), VEGF (clone VG-1; Santa Cruz), and ERG (clone EP11; CellMarque) were applied in dilutions ranging from 1/100 to 1/500 according to the manufacturer's instructions. Nuclear staining of at least 1% was interpreted as positive for ER and PR. Ki-67 expression of 14% or more was considered positive. As previously described,15 VEGF, ERG, and CD34 immunostaining were assessed semi-quantitatively using the Allred scoring system. Two groups were stratified based on the level of expression (low versus high VEGF, ERG, and CD34 expression groups).
Statistical Analysis
Statistical analysis was performed using the SPSS software, version 26.0 (IBM Corp., Armonk, NY, USA). The association between qualitative SMI parameters, histological grade, molecular markers, and ALNM status was tested using the Pearson chi-square or Fisher's exact test. The Mann–Whitney test was used to compare the differences in the vascular index among subgroups based on histological characteristics and ALNM. Subgroup analysis was also performed according to lesion size (<20 or ≥20 mm). A P value <.05 was deemed to indicate a statistically significant difference.
Results
Clinical and Histopathological Characteristics
Our study included 54 female patients with histopathologically confirmed IDC (mean age 49.5 ± 14.8 years, range 20–86 years). The average tumor size was 19.9 ± 8.7 mm. Steroid receptor status (ER and PR) was positive for 83.3% (45/54) and 90.7% (49/54) of the cases, respectively. Twenty-four patients (44.4%) who underwent preoperative FNAB and/or sentinel lymph node biopsy (SLNB) had ALNM. Among 54 cases, 57.4% (n = 31) of the tumors were high grade, 46.3% (n = 25) were Ki-67 positive, while only 7.4% (4/54) were Her-2 positive (Table 1).
| Number (%) | |
|---|---|
| Age (years) (mean ± SD) | 49.5 ± 14.8 |
| Lesion size (mm) (mean ± SD) | 19.9 ± 8.7 |
| Axillary lymph node metastasis | |
| Absent | 30 (55.6) |
| Present | 24 (44.4) |
| Histological grade | |
| Low | 23 (42.6) |
| High | 31 (57.4) |
| ER status | |
| Negative | 9 (16.7) |
| Positive | 45 (83.3) |
| PR status | |
| Negative | 5 (9.3) |
| Positive | 49 (90.7) |
| Her-2 receptor status | |
| Negative | 50 (92.6) |
| Positive | 4 (7.4) |
| Ki-67 index | |
| <14% | 29 (53.7) |
| >14% | 25 (46.3) |
- IDC, invasive ductal carcinoma; ER, estrogen receptor; PR, progesterone receptor.
VEGF and ERG expression was predominantly observed in the peritumoral region, with 92.5% (50/54) and 90.7% (49/54) of cases showing high peritumoral VEGF and ERG expression, respectively. In contrast, only 9.2% (5/54) and 25.9% (14/54) of the cases had high intratumoral VEGF and ERG expression, respectively. On the other hand, CD34 expression was higher in the intratumoral compartment, where 74% (40/54) of the cases showed high intratumoral CD34 expression, while high peritumoral CD34 expression was observed only in 3.7% (2/54) of the cases (Figure 3).
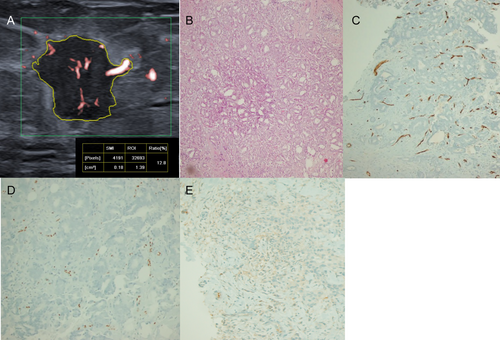
Superb Microvascular Imaging Findings
The mean vascular index of IDCs in the study was 6.8 ± 6.2 (range 0–25.6). A total of 34 tumors (62.9%) were classified as highly vascularized (score 2–3) based on Adler's classification. The majority of lesions had complex vessel morphology (36/54, 66.6%), central vascularity (39/54, 72.2%), and penetrating vessels (34/54, 62.9%) on SMI (Table 2).
| Parameters | Number (%) |
|---|---|
| Shape | |
| Oval/round | 23 (42.6) |
| Irregular | 31 (57.4) |
| Margin | |
| Circumscribed | 15 (27.8) |
| Not circumscribed | 39 (72.2) |
| Orientation | |
| Parallel | 20 (37.0) |
| Anti-parallel | 34 (63.0) |
| Echogenicity | |
| Hypoechoic | 47 (87.0) |
| Iso-hyperechoic | 7 (13.0) |
| Acoustic shadowing | |
| Absent | 30 (55.6) |
| Present | 24 (44.4) |
| Adler's score | |
| 0–1 (low vascularized) | 20 (37.0) |
| 2–3 (highly vascularized) | 34 (63.0) |
| Vessel morphology | |
| Simple | 18 (33.3) |
| Complex | 36 (66.7) |
| Vessel distribution | |
| Peripheral | 15 (27.8) |
| Central | 39 (72.2) |
| Penetrating vessels | |
| Absent | 20 (37.0) |
| Present | 34 (63.0) |
| Vascular index (mean ± SD) | 6.8 ± 6.2 |
The Association of SMI Parameters With Clinical and Histological Variables
IDCs with higher expression of VEGF in the peritumoral region showed higher vascular (7 ± 6.4 [95% CI 5.2–8.8] versus 3.7 ± 0.9 [95% CI 2.3–5.2], P = .003) on SMI. Likewise, high peritumoral ERG expression was linked to higher vascular index (7.2 ± 6.3 [95% CI 5.4–9.0] versus 2.4 ± 1 [95% CI 1.1–3.8], P < .001), complex vessel morphology (66.7% versus 20%, P = .024), penetrating vessels (63% versus 20%, P = .037), and central vascularity (77.6% versus 20%, P = .006). Tumors with higher intratumoral ERG expression demonstrated a more complex vessel morphology on SMI (85.7% versus 60%, P = 047). SMI parameters did not show any significant difference between low- and high-CD34-expressing tumors (P > .05) (Table 3).
| Adler's score, n (%) | P | Morphology, n (%) | P | Penetrating vessel, n (%) | P | Distribution, n (%) | P | Mean Vascular index (±SD) [95%CI lower–upper] | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 2–3 | Simple | Complex | Absent | Present | Peripheral | Central | |||||||
| Intratumoral | .60 | .66 | .91 | .44 | .98 | |||||||||
| CD34 (−) (n = 14) | 6 (42.9) | 8 (57.1) | 4 (28.6) | 10 (71.4) | 5 (25.7) | 9 (64.3) | 5 (35.7) | 9 (64.3) | 6.7 ± 6.9 [2.7–10.8] | |||||
| CD34 (+) (n = 40) | 14 (35) | 26 (65) | 14 (35) | 26 (65) | 15 (37.5) | 25 (62.5) | 10 (25) | 30 (75) | 6.7 ± 6 [4.8–8.7] | |||||
| Peritumoral | .27 | .31 | .27 | .37 | ||||||||||
| CD34 (−) (n = 52) | 20 (38.5) | 32 (61.5) | 18 (34.6) | 34 (65.4) | 20 (38.5) | 32 (61.5) | 15 (28.8) | 37 (71.2) | 6.7 ± 6.2 [4.9–8.4] | .59 | ||||
| CD34 (+) (n = 2) | 0 (0) | 2 (100) | 0 (0) | 2 (100) | 0 (0) | 2 (100) | 0 (0) | 2 (100) | 9.1 ± 8.2 [−65.2–83.4] | |||||
| Intratumoral | .41 | .74 | .89 | .55 | ||||||||||
| VEGF (−) (n = 49) | 19 (38.8) | 30 (61.2) | 16 (32.7) | 33 (67.3) | 18 (36.7) | 31 (63.3) | 13 (26.5) | 36 (73.5) | 7 ± 6.4 [5.2–8.9] | .32 | ||||
| VEGF (+) (n = 5) | 1 (20) | 4 (80) | 2 (40) | 3 (60) | 2 (40) | 3 (60) | 2 (40) | 3 (60) | 4.1 ± 3.5 [−0.2–8.4] | |||||
| Peritumoral | .60 | .14 | .14 | .20 | ||||||||||
| VEGF (−) (n = 4) | 1 (25) | 3 (75) | 0 (0) | 4 (100) | 0 (0) | 4 (100) | 0 (0) | 4 (100) | 3.7 ± 0.9 [2.3–5.2] | .003 | ||||
| VEGF (+) (n = 50) | 19 (38) | 31 (62) | 18 (36) | 32 (64) | 18 (36) | 32 (64) | 15 (30) | 35 (70) | 7 ± 6.4 [5.2–8.8] | |||||
| Intratumoral | .60 | .047 | .16 | .19 | ||||||||||
| ERG (−) (n = 40) | 14 (35) | 26 (65) | 16 (40) | 24 (60) | 17 (42.5) | 23 (57.5) | 13 (32.5) | 27 (67.5) | 6.7 ± 6.5 [4.7–8.9] | .98 | ||||
| ERG (+) (n = 14) | 6 (42.9) | 8 (57.1) | 2 (14.3) | 12 (85.7) | 3 (21.4) | 11 (78.6) | 2 (14.3) | 12 (85.7) | 6.7 ± 5.5 [3.6–9.9] | |||||
| Peritumoral | .41 | .024 | .037 | .006 | ||||||||||
| ERG (−) (n = 5) | 1 (20) | 4 (80) | 4 (80) | 1 (20) | 4 (80) | 1 (20) | 4 (80) | 1 (20) | 2.4 ± 1 [1.1–3.8] | <.001 | ||||
| ERG (+) (n = 49) | 19 (38.8) | 30 (61.2) | 14 (28.6) | 35 (71.4) | 16 (32.7) | 33 (67.3) | 11 (22.4) | 38 (77.6) | 7.2 ± 6.3 [5.4–9.0] | |||||
- Note that (−) refers to absent or low level of expression, while (+) corresponds to medium-to-high levels of expression. Bold values indicate statistical significance (P < .05). SD, standard deviation; CI, confidence interval.
In patients with positive ALNM, primary tumors were found to have a higher vascular index (10 ± 7.6 [95%CI 6.7–13.2] versus 4.2 ± 3 [95%CI 3.1–5.3], P < .001), complex vessel morphology (83.3% versus 53.3%, P = .020), and penetrating vessels (63.2% versus 50%, P = .027), compared to the ALNM-negative cases (Table 4). Subgroup analysis according to lesion size, histological grade, hormone receptor status, and Ki-67 expression level showed no significant difference in SMI parameters between groups (P > .05).
| SMI Parameters | ALNM (−) | ALNM (+) | P |
|---|---|---|---|
| (n = 30) | (n = 24) | ||
| Adler's score | .614 | ||
| 0–1 | 12 (40%) | 8 (33.3%) | |
| 2–3 | 18 (60%) | 16 (66.7%) | |
| Vessel morphology | .020 | ||
| Simple | 14 (46.7%) | 4 (16.8%) | |
| Complex | 16 (53.3%) | 20 (83.3%) | |
| Vessel distribution | .308 | ||
| Peripheral | 10 (33.3%) | 5 (20.8%) | |
| Central | 20 (66.7%) | 19 (79.2%) | |
| Penetrating vessels | .027 | ||
| Absent | 15 (50.0%) | 5 (20.8%) | |
| Present | 15 (50.0%) | 19 (79.1%) | |
| Vascular index (mean ± SD) | 4.2 ± 3.0 | 10.0 ± 7.6 | <.001 |
- Bold values indicate statistical significance (P < .05).
The Association of Markers of Angiogenesis With Clinical and Other Histological Features
The presence of ALNM was associated with a higher ERG expression in the peritumoral region (100% versus 83.3%, P = .045) (Table 5). Subgroup analysis according to lesion size, histological grade, hormone receptor status, and Ki-67 expression level revealed no significant difference in either VEGF or CD34 expression between groups (P > .05).
| Expression levels | ALNM (−) | ALNM (+) | P |
|---|---|---|---|
| (n = 30) | (n = 24) | ||
| Intratumoral | .890 | ||
| CD34 (−) | 8 (26.7%) | 6 (25.0%) | |
| CD34 (+) | 22 (73.3%) | 18 (75.0%) | |
| Peritumoral | 1.000 | ||
| CD34 (−) | 29 (96.7%) | 23 (95.8%) | |
| CD34 (+) | 1 (3.3%) | 1 (4.2%) | |
| Intratumoral | .646 | ||
| VEGF (−) | 28 (93.3%) | 21 (87.5%) | |
| VEGF (+) | 2 (6.7%) | 3 (12.5%) | |
| Peritumoral | .120 | ||
| VEGF (−) | 4 (13.3%) | 0 (0.0%) | |
| VEGF (+) | 26 (86.7%) | 24 (100.0%) | |
| Intratumoral | |||
| ERG (−) | 22 (73.3%) | 18 (75.0%) | .890 |
| ERG (+) | 8 (26.7%) | 6 (25.0%) | |
| Peritumoral | |||
| ERG (−) | 5 (16.7%) | 0 (0.0%) | .045 |
| ERG (+) | 25 (83.3%) | 24 (100.0%) |
- Note that (−) refers to absent or low level of expression, while (+) corresponds to medium-to-high levels of expression. Bold values indicate statistical significance (P < .05).
Discussion
In this prospective study, we explored the relationship between non-invasive sonographic microvascular imaging parameters, specifically using SMI, and histologic markers of angiogenesis (VEGF, ERG, and CD34) in patients with IDC. The findings suggest that higher neo-angiogenic activity, characterized by increased expression of angiogenic factors in the tumor microenvironment, is associated with specific vascular patterns, particularly higher vascular index, complex vessel morphology, penetrating vessels, and central vascularity on SMI, highlighting the potential utility of SMI as a non-invasive imaging tool for assessing tumor angiogenesis in real time.
Angiogenesis plays a fundamental role in tumor growth and metastatic spread by providing essential oxygen and nutrients.4 Tumors can induce angiogenesis by secreting various pro-angiogenic factors. VEGF, ERG, and CD34 play integral roles in vascular formation and stability within the tumor microenvironment. VEGF is a potent driver of angiogenesis, stimulating endothelial cell division, migration, and the formation of new blood vessels, thereby promoting increased blood supply to tumors under hypoxic conditions.16, 17 ERG, a member of the ETS transcription factor family, regulates endothelial cell functions and enhances VEGF-mediated angiogenesis by stabilizing nascent vessels and participating in the VEGF–VE-cadherin signaling pathway, which is critical for endothelial survival.8, 18 Meanwhile, CD34 is a cell surface glycoprotein and a common marker for endothelial progenitor cells and hematopoietic stem cells.19 Tumor MVD, measured as “vascular hot spots” after IHC staining with endothelial markers like CD34, CD31, or anti-Factor VIII-related antigen, has been evaluated as an indicator of the extent of angiogenesis in tissue specimens and serves as a quantitative benchmark for the measurement of tumoral angiogenesis.5, 20 Elevated VEGF expression and MVD were shown to be associated with poorer overall and disease-free survival in patients with breast cancer.5, 7, 21-23 Additionally, there are various reports linking these markers of angiogenesis with more aggressive tumor behavior such as ALNM and higher histologic grade.24 However, histopathological quantification of tumoral angiogenesis is limited by its invasive nature and may not fully capture the dynamic vascular architecture in vivo.
In recent years, color Doppler US (CD-US), power Doppler US (PD-US), contrast-enhanced ultrasound (CE-US), and dynamic contrast magnetic resonance imaging (DCE-MRI) have been among the imaging modalities that can be used to evaluate the vascularity of tumor tissue noninvasively.25-28 Several studies reported a correlation between tumor MVD by IHC analysis and perfusion parameters obtained through CE-US or DCE-MRI in breast tumors.26, 27, 29 However, both techniques require intravenous contrast agent administration, which can cause allergic reactions, incur additional costs, and consume additional time. SMI is an innovative non-contrast technique that uses novel algorithms to reduce motion artifacts, enabling the visualization of tiny blood vessels, vascularization patterns, and subtle blood supply increases in tumor tissue, superior to CD-US and PD-US.11 The features that indicate malignancy or aggressive behavior in SMI are branching and penetrating vessel morphology, the presence of central vessels, and a higher vascular index. A recent study by Zhang et al has shown that the vascular index measured via SMI was significantly correlated with higher histologic grade and Ki-67 index,30 while Bulut et al indicated that SMI, combined with shear wave elastography, effectively predicted the presence of ALNM in patients with breast cancer, suggesting that higher vascularity in SMI is linked to more aggressive tumor phenotypes and poorer outcomes.31 Likewise, there are studies suggesting that SMI can be used as an indicator of neoadjuvant therapies in the evaluation of early anti-angiogenic effects in real time.32, 33 However, the potential association between SMI parameters and histological markers of tumoral angiogenesis in IDC has not been previously studied.
In this study, we observed that IDCs with higher expression of VEGF and ERG exhibited more aggressive vascular features on SMI, such as increased vascular index, complex vessel morphology, penetrating vessels, and central vascularity. Furthermore, the presence of ALNM was linked to both aggressive SMI features and higher peritumoral ERG expression. Notably, CD34 expression did not show a significant correlation with SMI parameters. This may suggest that while CD34 is typically a reliable marker for measuring the quantity of microvessels in tissue samples and provides a robust quantitative assessment for the static end-stage view of angiogenesis, SMI might also independently offer dynamic insights into the in vivo evolving tumoral vascular architecture, such as flow patterns and vessel complexity, which may be more directly reflected in the expression levels of pro-angiogenic factors such as VEGF and ERG. However, further research with larger sample sizes is warranted to elucidate the interaction between traditional markers of MVD, other pro-angiogenic factors, and the dynamic vascular architecture observed through SMI. Interestingly, we also found aggressive SMI features were more widely associated with high peritumoral expression of VEGF and ERG, compared to expression levels in the intratumoral compartment, possibly due to the fact that angiogenesis is generally more active at the tumor periphery than in the center, driven by several factors such as hypoxia gradient and up-regulation of pro-angiogenic factors in peritumoral tissue.34, 35
Several limitations of the study should be noted. First, the study sample size was relatively small, and larger cohorts are needed to validate these findings. SMI, like other sonographic techniques, has the potential to induce variability because it is user-dependent. In our study, the evaluation of images was performed according to the consensus, and intra-interobserver variability was not examined. The significant overlap in CIs observed for certain parameters in this study may reflect the small sample size, variability in measurements, or inherent biological variability, which limits the precision of the findings and should be addressed in future studies with larger cohorts. Additionally, this study only included patients with IDC, limiting the generalizability of the results to other subtypes of breast cancer. Furthermore, the IHC analyses were conducted on core biopsy specimens, which may not fully represent the entire tumor's angiogenic profile. It would have been more optimal to evaluate the expression levels of angiogenesis markers on a surgical specimen. However, since some of our patients received neoadjuvant treatment, their angiogenetic structure could potentially be influenced. For this reason, pathological analysis was performed on biopsy specimens at the time of diagnosis. Lastly, this study did not evaluate the longitudinal changes in SMI parameters over time, which could provide valuable information about the dynamic nature of angiogenesis in response to treatment.
Our study was a pilot study. In the future, more comprehensive studies with larger sample sizes and multivariate regression models with various factors (eg, a model that predicts the likelihood of LN involvement based on a combination of SMI, VEFG/ERG expression, and clinical factors) are needed to improve our findings.
Conclusion
This study demonstrates that tumors with higher angiogenic activity, particularly those expressing higher levels of VEGF and ERG in the peritumoral region, exhibit distinct microvascular patterns on SMI, such as increased vascular index, complex vessel morphology, and penetrating vessels. These SMI features are also associated with more aggressive tumor characteristics, including ALNM. Future studies should focus on validating these findings in larger cohorts and exploring the clinical implications of SMI in monitoring treatment response, particularly in patients receiving anti-angiogenic therapies.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




