Neurosonographic Measurements of the Fetal Anterior Complex in Singleton Pregnancies
We thank Ellen Knapp, PhD, from Edanz (https://edanz.com/ac) for editing a draft of this manuscript.
This research was supported by the grants from the Faculty of Medicine Ramathibodi Hospital, Mahidol University, Bangkok, Thailand (grant RF_65049). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abstract
Objective
To construct reference ranges of the fetal cerebral anterior complex, including ventricular index (VI), anterior horn of lateral ventricle width (AW), and cavum septi pellucidi (CSP) width, as a function of gestational age (GA), in Thai fetuses.
Methods
Low-risk pregnancies were recruited to measure fetal anterior complex on axial transventricular and coronal transcaudate planes using transabdominal ultrasound. The downside and upside hemisphere were defined as cerebral hemisphere located distal and proximal to the transducer, respectively. The five variables, downside/upside VI, downside/upside AW and CSP width, were measured from each fetus. Best-fit models in predicting mean and standard deviation for each value as a function of GA were constructed, using regression analysis. Distributions of Z-scores of all values based on GA were created to evaluate the fitness of models. Intraclass correlation coefficients were used to assess inter-/intraobserver variability.
Results
A total of 395 fetuses were measured for anterior complex. All parameters changed with GA with quadratic function. The models for predicting means and standard deviation of the five parameters as well as percentile charts were created. All models were proven well-fitted. The intra-/interobserver reliability coefficients of all values showed excellent agreement.
Conclusion
The reference ranges of the fetal anterior complex, including VI, AW, and CSP, in axial transventricular and coronal transcaudate planes have been established and available for clinical use.
Abbreviations
-
- AW
-
- anterior horn of the lateral ventricle width
-
- CNS
-
- central nervous system
-
- CSP
-
- cavum septi pellucidi
-
- GA
-
- gestational age
-
- IHF
-
- interhemispheric fissure
-
- VI
-
- ventricular index
Central nervous system (CNS) malformations which can cause perinatal death or childhood disability, are one of the most common congenital abnormalities detected prenatally.1, 2 The detection of fetal structural brain anomalies enables obstetricians to not only plan antenatal management, but also provide important information for parental counseling. To improve the prenatal diagnosis of fetal CNS malformations, the International Society of Ultrasound in Obstetrics and Gynecology has introduced routine ultrasound screening of the fetal CNS at mid-trimester in low-risk pregnant women, together with targeted neurosonography in high-risk pregnant women.3-5
The anterior complex of the fetal brain is a group of anatomical structures comprising the cavum septi pellucidi (CSP), anterior horns of the lateral ventricle, genu of the corpus callosum, pericallosal sulcus, and the anterior part of interhemispheric fissure (IHF). In standard transabdominal ultrasound in the axial transventricular plane, the anterior complex can be identified ~80–100% of the time in the second and third trimesters.6-8 The normal anterior horns of the lateral ventricle have a well-demarcated lateral wall, and appear as two comma-shaped or triangular structures. These horns are separated medially by the CSP, which is a square-shaped or triangular fluid-filled cavity.6
Integrating anterior complex assessment into standard CNS scans has the potential to improve the prenatal diagnosis of midline and cortical malformations of the fetal brain.6, 9-13 Anterior complex abnormalities can be evaluated subjectively by assessing their morphology. Dysmorphic features of the anterior complex, such as fused anterior horns of the lateral ventricle, quadrangular-shaped anterior horns of the lateral ventricle, an atypical-shaped CSP, or a distorted IHF, aid in the diagnosis of holoprosencephaly, schizencephaly, heterotopia, and partial agenesis of the corpus callosum, respectively.6, 10 In addition, quantitative measurements of the anterior complex have been examined. The reference ranges of these structures, such as the anterior horn of the lateral ventricle width (AW), CSP width, and ventricular index (VI) (defined as the distance between the lateral wall of the anterior horn of the lateral ventricle and the IHF), in the fetus and preterm neonate have been reported in several studies.8, 14-17 The combination of subjectively visualizing dysmorphology together with objectively measuring the structures in the anterior complex compared with normal reference curves may facilitate the identification of prenatal CNS malformations.
However, among reported reference curves, there is variation in the measurement techniques and conflicting outcomes.8, 14-17 Most studies did not include a standard deviation model for calculating Z scores, which is required for clinical applications in assessing the anterior complex.8, 14, 17 Moreover, some studies have the limitation of no neonatal assessment to confirm normal outcomes and not providing examples of abnormal cases to validate the curve.8, 16, 17 In addition, intraobserver and interobserver reliabilities of each measurement value are scarce.8, 14, 17
Therefore, the primary objective of this study was to construct reference ranges of the anterior complex, including the VI, AW, and CSP width, based on gestational age (GA) in Thai fetuses. The secondary objective was to compare measured values between different sides of the structures (downside vs upside of the probe, which were defined as cerebral hemisphere located distal and proximal to the transducer, respectively, and right vs left hemisphere), different planes (axial transventricular vs coronal transcaudate planes), and different fetal sexes.
Materials and Methods
This prospective, cross-sectional study was conducted between October 2021 and September 2022 at the Antenatal Care Clinic, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand. The protocol was approved by the Ramathibodi Hospital Institutional Review Board (COA, MURA2021/852) and complied with the Declaration of Helsinki. The study was registered with the Thai Clinical Trials Registry, TCTR20220129001. All pregnant women were consecutively counseled and enrolled with written informed consent.
The inclusion criteria were as follows: 1) a singleton pregnancy; 2) maternal age of 18 years or older; 3) GA between 17 and 37 weeks (based on a well-defined last menstrual period or fetal biometry by ultrasound scanning in the first half of pregnancy); 4) low-risk pregnancy without known medical problems (eg, chronic hypertension, diabetes mellitus, thyroid disease, renal disease, and autoimmune disease); and 5) no use of tobacco, alcohol drinking, or illicit drug use. The exclusion criteria included: 1) subsequent medical or obstetrical complications, such as preterm labor, pregnancy-induced hypertension, or oligohydramnios; 2) small-for-gestational age (estimated fetal weight of <10th percentile for GA) and large-for-gestational age (>90th percentile for GA); 3) fetal structural or chromosomal abnormalities diagnosed prenatally or postnatally; 4) poor quality imaging; 5) unknown pregnancy outcomes; and 6) women who declined to participate.
All participants were evaluated by ultrasound, which was standardized and performed onsite by trained maternal fetal medicine (MFM) fellows (JK, WL) and MFM staff members (WD, CT) using grayscale two-dimensional ultrasound. A 3- to 5-MHz transabdominal curvilinear transducer connected to a Voluson E10 (GE Healthcare, Zipf, Austria) was used in all ultrasound examinations. All participants underwent fetal ultrasound for documentation of fetal biometry and presentation, fetal weight, amount of amniotic fluid, and placental location. Standard fetal abnormality screening was also conducted on fetuses between the ages of 18 and 22 weeks. All data were recorded in the record form.
The fetal anterior complex was clearly identified at the axial transventricular plane.4 The criteria for axial transventricular plane consisted of the falx cerebri at the midline and perpendicular to the ultrasound beam, symmetrical hemispheres, visible anterior and posterior horns of the lateral ventricles, no visible cerebellum, and the presence of the CSP. Images of the anterior complex were subsequently magnified by 30%. The VI was measured at the outermost of the lateral wall of the anterior horn, by positioning the calipers outside the echoes generated by the lateral walls of the anterior horn to midline of the IHF and perpendicular to the IHF. On the same image, measurement of the AW was performed at the widest diameter of the anterior horn, and the calipers were placed on the inner edge to inner edge of the anterior horn and perpendicular to the ventricular cavity. The CSP width was measured from the inner edge to the inner edge of the CSP at the maximal transverse diameter of the CSP and perpendicular to the IHF (Figure 1). Three sets of acquisitions and measurements at downside and upside hemisphere were obtained for each fetus. Downside and upside hemisphere were defined as cerebral hemisphere located distal and proximal to the transducer, respectively.
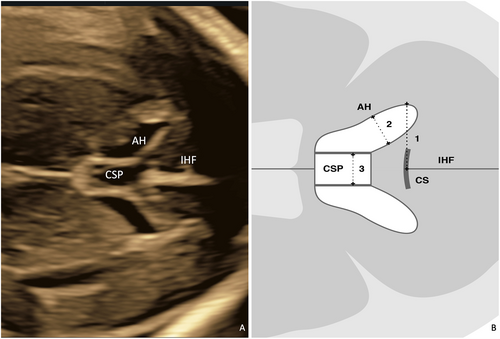
In the same participant, the transducer was then rotated 90° to obtain the coronal transcaudate plane.5 In this plane, the criteria for acquisition were visualization of the falx cerebri at the midline and perpendicular to the ultrasound beam, symmetrical hemispheres, the anterior horn of the lateral ventricles, and caudate nuclei, the corpus callosum and CSP, with no visible cerebellum (Figure 2). Three sets of acquisitions and measurements of the downside/upside VI, downside/upside AW, and CSP width were performed using the criteria listed above.

Each participant was scanned only once during the study. Three sets of acquisitions and measurements were obtained for each fetus. All sonographers were blinded to their own each measurement in the three acquisitions. The mean of the measured values was calculated and recorded. Intraobserver variability was calculated. Moreover, two of the four operators performed acquisitions and measurements in a subset of 27 fetuses. The sonographers were blinded to the other sonographers' measurements. The mean of the measured values was calculated and recorded. The interobserver variability was calculated in a subset of 27 pregnant women.
During the study period, fetuses with CNS abnormalities were collected, for subsequent use as an example of clinical application of the constructed reference ranges. Demographic data such as the maternal age at the time of the ultrasound and the pre-pregnancy body mass index were reviewed and documented in the record form at the time of recruitment. Maternal and neonatal outcomes were followed and collected.
Statistical Analysis
Parametric continuous variables are expressed as the mean ± standard deviation and were compared using Student's t-test. Categorical variables were defined as the number (%). P < .05 was considered statistically significant. All statistical analyses were calculated using the statistical package for the social sciences (SPSS) software version 26.0 (IBM Corp Released 2019, IBM SPSS Statistics for Windows, Version 26.0). According to Royston and Wright,18 best-fit regression equations of the means and standard deviations (SDs) for the VI, AW, and CSP width were generated using GA as an independent variable. The regression models were separately created for the means and SDs of the anterior complex dimensions as functions of GA and used for construction of reference ranges. The Z-scores were tested for a normal distribution and the goodness of fit of these regression models. Based on the best-fit equation and the predicted SD, predictive values for 5th, 10th, 50th, 90th, and 95th percentile ranges of anterior complex dimensions were constructed. Intraobserver and interobserver variability were calculated with the intraclass correlation coefficient technique.
Results
During the study period, 404 Thai low-risk pregnant women were enrolled in the study. Of these, nine (2.2%) pregnancies were excluded from the analysis because of poor-quality imaging (2%) and fetal growth restriction (0.2%). A total of 395 women with complete data were available for the analysis.
The mean maternal age and mean pre-pregnancy body mass index were 30.6 ± 5.5 years and 22.5 ± 3.9 kg/m2, respectively. Slightly more than half (53.9%) of the women were nulliparous. At the scan, nearly three quarters (74.9%) of the fetuses were in cephalic presentation. The frequency of placentas located anteriorly (46.8%) and posteriorly (53.2%) was comparable. The mean GA at delivery and mean birth weight was 38.4 ± 1.4 weeks and 3122 ± 429 g, respectively. The frequency of male (55.4%) and female (44.3%) newborns were comparable.
On the axial plane, the downside VI and AW were significantly larger than the upside VI and AW (P < .001; P < .001, respectively). On the coronal plane, the downside VI and AW were also significantly greater than the upside VI and AW (P = .001; P < .001, respectively). In addition, when comparing the downside and upside VI on the axial versus the coronal planes, they were significantly different (downside: P < .001 and upside: P < .001). However, the measurement values of downside and upside AW and the CSP width on the axial versus those on the coronal planes were not significantly different (P = .613, P = .633, P = .715, respectively). There were no significant differences between the measurements obtained on the right vs those obtained on the left side of the fetal brain (P > .05 in all variables) and also no significant difference between fetal sexes (P > .05 in all variables).
All variables show normal distribution, based on non-significant results of Kolmogorov–Smirnov test and Shapiro–Wilk test in nearly all values. The mean VI, AW, and CSP width showed a significant correlation with GA (R2: .7, .3, and .6, respectively; and P-value <.001 in all variables). The quadratic and linear regression equations contributed to a good fit for the predicted mean and predicted SD of all measurement values. The best-fitted regression models in predicting means and SDs of downside and upside VI, downside and upside AW and CSP width on the axial transventricular plane as well as downside and upside VI on the coronal transcaudate plane are shown in Table 1.
| Best-Fitted Models | R2 | P-Value | |
|---|---|---|---|
| Axial plane | |||
| Downside VI | |||
| Mean | 9.353 − 0.212 × GA + 0.008 × GA2 | .722 | <.001 |
| SD | −0.042 + 0.034 × GA | .084 | <.001 |
| Upside VI | |||
| Mean | 9.513 − 0.236 × GA + 0.009 × GA2 | .734 | <.001 |
| SD | −0.271 + 0.042 × GA | .126 | <.001 |
| Downside AW | |||
| Mean | 10.179 − 0.521 × GA + 0.009 × GA2 | .326 | <.001 |
| SD | 1.363 − 0.031 × GA | .133 | <.001 |
| Upside AW | |||
| Mean | 10.650 − 0.560 × GA + 0.009 × GA2 | .334 | <.001 |
| SD | 1.559 − 0.038 × GA | .177 | <.001 |
| CSP width | |||
| Mean | −10.061 + 0.969 × GA − 0.015 × GA2 | .626 | <.001 |
| SD | 0.245 + 0.020 × GA | .037 | <.001 |
| Coronal plane | |||
| Downside VI | |||
| Mean | 10.121 − 0.248 × GA + 0.009 × GA2 | .732 | <.001 |
| SD | −0.026 + 0.031 × GA | .081 | <.001 |
| Upside VI | |||
| Mean | 10.758 − 0.3102 × GA + 0.010 × GA2 | .739 | <.001 |
| SD | −0.135 + 0.037 × GA | .109 | <.001 |
- AW, anterior horn of lateral ventricle width (mm); CSP, cavum septi pellucidi (mm); GA, gestational age (weeks); SD, standard deviation; VI, ventricular index (mm).
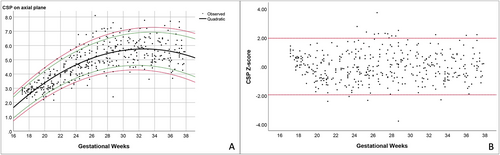
Based on the best-fitted models mentioned above, the predicted means and various percentile ranges throughout pregnancy were calculated and plotted (Figures 3-5). The percentile charts of downside and upside VI, downside and upside AW and CSP width on the axial transventricular plane, as well as downside and upside VI on the coronal transcaudate plane as a function of GA, are shown in Tables 2–5.
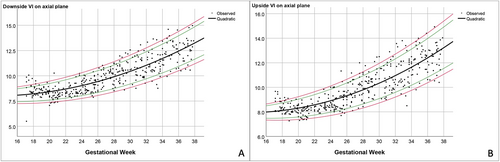
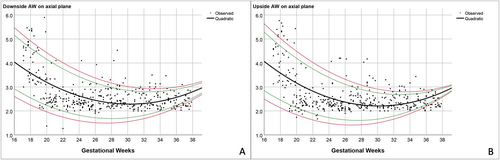
| GA | n | Downside VI | Upside VI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5th | 10th | 50th | 90th | 95th | 5th | 10th | 50th | 90th | 95th | ||
| 17 | 7 | 7.26 | 7.46 | 8.16 | 8.85 | 9.05 | 7.32 | 7.48 | 8.05 | 8.63 | 8.79 |
| 18 | 23 | 7.28 | 7.49 | 8.22 | 8.95 | 9.16 | 7.32 | 7.50 | 8.11 | 8.73 | 8.90 |
| 19 | 15 | 7.32 | 7.54 | 8.31 | 9.08 | 9.30 | 7.34 | 7.53 | 8.20 | 8.87 | 9.06 |
| 20 | 24 | 7.37 | 7.61 | 8.42 | 9.24 | 9.47 | 7.38 | 7.58 | 8.31 | 9.03 | 9.24 |
| 21 | 24 | 7.44 | 7.68 | 8.54 | 9.40 | 9.64 | 7.43 | 7.65 | 8.42 | 9.19 | 9.41 |
| 22 | 14 | 7.53 | 7.79 | 8.70 | 9.61 | 9.86 | 7.51 | 7.75 | 8.58 | 9.41 | 9.65 |
| 23 | 21 | 7.64 | 7.92 | 8.87 | 9.82 | 10.09 | 7.61 | 7.86 | 8.75 | 9.63 | 9.89 |
| 24 | 25 | 7.77 | 8.06 | 9.06 | 10.05 | 10.34 | 7.72 | 7.99 | 8.93 | 9.88 | 10.14 |
| 25 | 20 | 7.90 | 8.20 | 9.23 | 10.27 | 10.56 | 7.84 | 8.12 | 9.11 | 10.10 | 10.38 |
| 26 | 21 | 8.03 | 8.34 | 9.41 | 10.48 | 10.79 | 7.96 | 8.25 | 9.29 | 10.33 | 10.62 |
| 27 | 16 | 8.19 | 8.51 | 9.63 | 10.75 | 11.06 | 8.11 | 8.42 | 9.51 | 10.60 | 10.91 |
| 28 | 24 | 8.39 | 8.73 | 9.89 | 11.05 | 11.39 | 8.30 | 8.63 | 9.78 | 10.92 | 11.25 |
| 29 | 23 | 8.60 | 8.94 | 10.15 | 11.36 | 11.70 | 8.50 | 8.84 | 10.04 | 11.24 | 11.59 |
| 30 | 10 | 8.81 | 9.17 | 10.41 | 11.66 | 12.02 | 8.70 | 9.06 | 10.31 | 11.56 | 11.92 |
| 31 | 20 | 9.06 | 9.43 | 10.73 | 12.03 | 12.40 | 8.95 | 9.32 | 10.63 | 11.94 | 12.32 |
| 32 | 18 | 9.31 | 9.69 | 11.03 | 12.37 | 12.75 | 9.19 | 9.58 | 10.94 | 12.30 | 12.69 |
| 33 | 17 | 9.58 | 9.98 | 11.36 | 12.74 | 13.13 | 9.46 | 9.86 | 11.28 | 12.69 | 13.10 |
| 34 | 19 | 9.86 | 10.26 | 11.69 | 13.11 | 13.52 | 9.73 | 10.15 | 11.62 | 13.08 | 13.50 |
| 35 | 15 | 10.17 | 10.59 | 12.06 | 13.53 | 13.95 | 10.04 | 10.47 | 12.00 | 13.52 | 13.95 |
| 36 | 15 | 10.48 | 10.92 | 12.43 | 13.94 | 14.37 | 10.36 | 10.80 | 12.38 | 13.95 | 14.40 |
| 37 | 18 | 10.85 | 11.29 | 12.85 | 14.41 | 14.85 | 10.72 | 11.18 | 12.81 | 14.45 | 14.91 |
| 38 | 6 | 11.07 | 11.53 | 13.11 | 14.70 | 15.15 | 10.94 | 11.42 | 13.09 | 14.75 | 15.23 |
- GA, gestational age (weeks); n, number; VI, ventricular index (mm).
| GA | n | Downside VI | Upside VI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5th | 10th | 50th | 90th | 95th | 5th | 10th | 50th | 90th | 95th | ||
| 17 | 7 | 7.56 | 7.77 | 8.50 | 9.22 | 9.43 | 7.59 | 7.78 | 8.43 | 9.09 | 9.27 |
| 18 | 23 | 7.57 | 7.79 | 8.55 | 9.31 | 9.52 | 7.58 | 7.78 | 8.47 | 9.16 | 9.36 |
| 19 | 15 | 7.60 | 7.83 | 8.63 | 9.43 | 9.66 | 7.59 | 7.80 | 8.54 | 9.28 | 9.49 |
| 20 | 24 | 7.65 | 7.89 | 8.73 | 9.57 | 9.81 | 7.61 | 7.84 | 8.63 | 9.41 | 9.64 |
| 21 | 24 | 7.71 | 7.96 | 8.84 | 9.71 | 9.96 | 7.66 | 7.89 | 8.72 | 9.55 | 9.79 |
| 22 | 14 | 7.81 | 8.07 | 8.99 | 9.91 | 10.17 | 7.73 | 7.98 | 8.86 | 9.75 | 10.00 |
| 23 | 21 | 7.91 | 8.19 | 9.15 | 10.11 | 10.39 | 7.82 | 8.09 | 9.02 | 9.95 | 10.22 |
| 24 | 25 | 8.04 | 8.33 | 9.33 | 10.34 | 10.62 | 7.93 | 8.21 | 9.20 | 10.18 | 10.46 |
| 25 | 20 | 8.17 | 8.46 | 9.51 | 10.55 | 10.84 | 8.05 | 8.34 | 9.37 | 10.40 | 10.69 |
| 26 | 21 | 8.30 | 8.60 | 9.68 | 10.76 | 11.06 | 8.17 | 8.48 | 9.55 | 10.61 | 10.92 |
| 27 | 16 | 8.47 | 8.78 | 9.90 | 11.01 | 11.33 | 8.33 | 8.65 | 9.77 | 10.88 | 11.20 |
| 28 | 24 | 8.67 | 9.00 | 10.16 | 11.32 | 11.65 | 8.53 | 8.87 | 10.03 | 11.20 | 11.53 |
| 29 | 23 | 8.88 | 9.22 | 10.42 | 11.62 | 11.96 | 8.74 | 9.09 | 10.31 | 11.52 | 11.87 |
| 30 | 10 | 9.10 | 9.45 | 10.69 | 11.92 | 12.28 | 8.96 | 9.32 | 10.58 | 11.84 | 12.20 |
| 31 | 20 | 9.36 | 9.72 | 11.01 | 12.29 | 12.65 | 9.23 | 9.60 | 10.92 | 12.23 | 12.60 |
| 32 | 18 | 9.61 | 9.99 | 11.31 | 12.63 | 13.01 | 9.49 | 9.88 | 11.24 | 12.60 | 12.98 |
| 33 | 17 | 9.90 | 10.28 | 11.64 | 13.01 | 13.39 | 9.78 | 10.18 | 11.59 | 13.00 | 13.40 |
| 34 | 19 | 10.18 | 10.58 | 11.98 | 13.38 | 13.78 | 10.08 | 10.49 | 11.95 | 13.40 | 13.82 |
| 35 | 15 | 10.51 | 10.92 | 12.36 | 13.80 | 14.21 | 10.42 | 10.85 | 12.35 | 13.85 | 14.28 |
| 36 | 15 | 10.84 | 11.26 | 12.74 | 14.22 | 14.64 | 10.76 | 11.20 | 12.76 | 14.31 | 14.75 |
| 37 | 18 | 11.21 | 11.65 | 13.17 | 14.70 | 15.13 | 11.16 | 11.62 | 13.22 | 14.82 | 15.28 |
| 38 | 6 | 11.45 | 11.90 | 13.45 | 15.00 | 15.44 | 11.41 | 11.88 | 13.51 | 15.15 | 15.61 |
- GA, gestational age (weeks); n, number; VI, ventricular index (mm).
| GA | n | Downside AW | Upside AW | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 5th | 10th | 50th | 90th | 95th | 5th | 10th | 50th | 90th | 95th | ||
| 17 | 7 | 2.38 | 2.68 | 3.75 | 4.82 | 5.13 | 2.26 | 2.60 | 3.76 | 4.92 | 5.26 |
| 18 | 23 | 2.24 | 2.54 | 3.58 | 4.62 | 4.92 | 2.13 | 2.45 | 3.58 | 4.70 | 5.02 |
| 19 | 15 | 2.10 | 2.38 | 3.38 | 4.38 | 4.67 | 1.98 | 2.29 | 3.36 | 4.44 | 4.75 |
| 20 | 24 | 1.96 | 2.23 | 3.19 | 4.15 | 4.43 | 1.84 | 2.13 | 3.16 | 4.19 | 4.49 |
| 21 | 24 | 1.85 | 2.11 | 3.04 | 3.96 | 4.23 | 1.73 | 2.01 | 3.00 | 3.99 | 4.27 |
| 22 | 14 | 1.74 | 1.99 | 2.87 | 3.75 | 4.00 | 1.62 | 1.89 | 2.82 | 3.75 | 4.02 |
| 23 | 21 | 1.65 | 1.89 | 2.73 | 3.57 | 3.81 | 1.54 | 1.79 | 2.67 | 3.55 | 3.81 |
| 24 | 25 | 1.58 | 1.81 | 2.61 | 3.41 | 3.64 | 1.48 | 1.71 | 2.54 | 3.38 | 3.61 |
| 25 | 20 | 1.54 | 1.76 | 2.52 | 3.29 | 3.50 | 1.44 | 1.66 | 2.45 | 3.24 | 3.46 |
| 26 | 21 | 1.51 | 1.72 | 2.45 | 3.18 | 3.39 | 1.42 | 1.63 | 2.38 | 3.12 | 3.34 |
| 27 | 16 | 1.50 | 1.69 | 2.39 | 3.08 | 3.28 | 1.41 | 1.61 | 2.31 | 3.01 | 3.21 |
| 28 | 24 | 1.50 | 1.68 | 2.33 | 2.98 | 3.17 | 1.42 | 1.61 | 2.25 | 2.90 | 3.08 |
| 29 | 23 | 1.51 | 1.69 | 2.30 | 2.91 | 3.09 | 1.45 | 1.62 | 2.22 | 2.82 | 2.99 |
| 30 | 10 | 1.55 | 1.71 | 2.29 | 2.86 | 3.02 | 1.50 | 1.65 | 2.21 | 2.76 | 2.92 |
| 31 | 20 | 1.61 | 1.76 | 2.29 | 2.82 | 2.97 | 1.57 | 1.71 | 2.21 | 2.71 | 2.86 |
| 32 | 18 | 1.67 | 1.82 | 2.31 | 2.80 | 2.94 | 1.65 | 1.78 | 2.24 | 2.69 | 2.82 |
| 33 | 17 | 1.76 | 1.89 | 2.35 | 2.80 | 2.93 | 1.76 | 1.87 | 2.28 | 2.68 | 2.80 |
| 34 | 19 | 1.86 | 1.98 | 2.40 | 2.81 | 2.93 | 1.88 | 1.98 | 2.34 | 2.70 | 2.80 |
| 35 | 15 | 1.99 | 2.10 | 2.47 | 2.85 | 2.96 | 2.02 | 2.11 | 2.42 | 2.73 | 2.82 |
| 36 | 15 | 2.13 | 2.22 | 2.56 | 2.90 | 2.99 | 2.18 | 2.26 | 2.52 | 2.78 | 2.85 |
| 37 | 18 | 2.30 | 2.38 | 2.68 | 2.97 | 3.05 | 2.37 | 2.43 | 2.64 | 2.85 | 2.91 |
| 38 | 6 | 2.41 | 2.48 | 2.75 | 3.02 | 3.10 | 2.50 | 2.55 | 2.73 | 2.91 | 2.96 |
- AW, anterior horn of lateral ventricle width (mm); GA, gestational age (weeks); n, number.
| GA | n | CSP Width | ||||
|---|---|---|---|---|---|---|
| 5th | 10th | 50th | 90th | 95th | ||
| 17 | 7 | 1.25 | 1.47 | 2.23 | 2.99 | 3.21 |
| 18 | 23 | 1.57 | 1.80 | 2.58 | 3.36 | 3.58 |
| 19 | 15 | 1.95 | 2.18 | 2.99 | 3.80 | 4.03 |
| 20 | 24 | 2.31 | 2.55 | 3.39 | 4.22 | 4.46 |
| 21 | 24 | 2.61 | 2.86 | 3.72 | 4.57 | 4.82 |
| 22 | 14 | 2.95 | 3.20 | 4.08 | 4.97 | 5.22 |
| 23 | 21 | 3.22 | 3.48 | 4.40 | 5.31 | 5.57 |
| 24 | 25 | 3.47 | 3.74 | 4.68 | 5.62 | 5.89 |
| 25 | 20 | 3.66 | 3.93 | 4.90 | 5.86 | 6.13 |
| 26 | 21 | 3.81 | 4.09 | 5.08 | 6.06 | 6.34 |
| 27 | 16 | 3.96 | 4.25 | 5.26 | 6.27 | 6.56 |
| 28 | 24 | 4.09 | 4.39 | 5.43 | 6.47 | 6.76 |
| 29 | 23 | 4.19 | 4.49 | 5.56 | 6.62 | 6.92 |
| 30 | 10 | 4.25 | 4.56 | 5.65 | 6.74 | 7.05 |
| 31 | 20 | 4.28 | 4.60 | 5.72 | 6.84 | 7.16 |
| 32 | 18 | 4.28 | 4.61 | 5.75 | 6.90 | 7.22 |
| 33 | 17 | 4.26 | 4.59 | 5.76 | 6.93 | 7.26 |
| 34 | 19 | 4.20 | 4.54 | 5.74 | 6.93 | 7.27 |
| 35 | 15 | 4.11 | 4.46 | 5.68 | 6.90 | 7.25 |
| 36 | 15 | 4.00 | 4.35 | 5.60 | 6.85 | 7.20 |
| 37 | 18 | 3.84 | 4.20 | 5.48 | 6.75 | 7.12 |
| 38 | 6 | 3.73 | 4.10 | 5.39 | 6.68 | 7.05 |
- CSP, cavum septi pellucidi (millimeters); GA, gestational age (weeks); n, number.
The goodness of fit for all equations was validated by the Q–Q plots and distributions of the Z-scores for each measurement. The Z-score values were symmetrically scattered within the line from −1.96 to 1.96 throughout gestation, indicating that all models are well-fitted (Figure 5B).
Intraobserver and interobserver variability for all measurements of the VI, AW, and CSP width was analyzed by the intraclass correlation coefficient, which showed good to excellent agreement (Table 6).
| Intraobserver Variability (95% CI) | Interobserver Variability (95% CI) | |
|---|---|---|
| Axial plane | ||
| Downside VI | 0.974 (0.969–0.978) | 0.927 (0.893–0.952) |
| Upside VI | 0.971 (0.965–0.975) | 0.925 (0.889–0.951) |
| Downside AW | 0.931 (0.918–0.942) | 0.914 (0.874–0.943) |
| Upside AW | 0.955 (0.946–0.962) | 0.940 (0.912–0.961) |
| CSP width | 0.978 (0.974–0.982) | 0.956 (0.934–0.971) |
| Coronal plane | ||
| Downside VI | 0.978 (0.973–0.981) | 0.918 (0.879–0.946) |
| Upside VI | 0.964 (0.958–0.970) | 0.908 (0.865–0.950) |
- Note: Intraclass correlation coefficient was analyzed, using two-way mixed effects model, where people effects are random and measures effects are fixed, and type C using a consistency definition.
- AW, anterior horn of lateral ventricle width; CI, confidence interval; CSP, cavum septi pellucidi; VI, ventricular index.
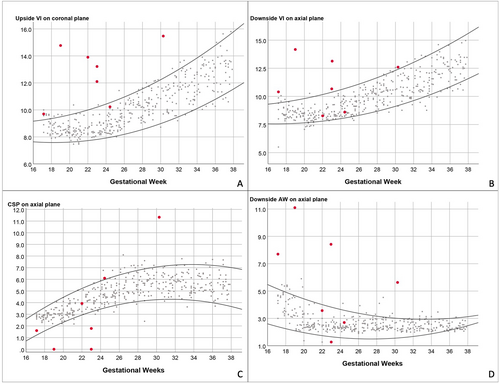
An example of the clinical application of the VI, AW, and CSP width reference ranges in fetuses with abnormal CNS malformations, including left parieto-occipital encephalocele, aqueductal stenosis, bilateral ventriculomegaly, left intraventricular subarachnoid cyst, right hemimegalencephaly, complete agenesis of the corpus callosum, and periventricular nodular heterotropia, is shown in Figure 6. In majority of cases, upside, downside VI values, and downside AW values greater than the 95th percentile of the normal range. Furthermore, the CSP was either absent or lower than the 5th percentile of the curve.
Discussion
Identification of the fetal cerebral anterior complex is achievable in the standard plane of transabdominal ultrasound.6-8 Moreover, part of the hemisphere proximal to the transducer can be analyzed via transventricular and transcaudate plane.12 The detection of abnormalities of structures contained in the anterior complex can be helpful in the diagnosis of midline and cortical malformations, such as holoprosencephaly, agenesis of the corpus callosum, schizencephaly, and heterotopia.6, 9-11 The combination of subjectively visualizing dysmorphology together with objectively measuring the structures in the anterior complex compared with normal reference curves may facilitate the identification of prenatal CNS malformations. In this study, we established reference values for the fetal cerebral anterior complex, including downside and upside VI, AW, and CSP width in the axial transventricular plane, as well as downside and upside VI in the coronal transcaudate plane, between 17 and 37 weeks in low-risk pregnant women.
All measurement values of the anterior complex showed a good correlation with GA, especially the VI and CSP (R2: .7 and .6, respectively). The mean VI increased with GA when measured in the axial and coronal planes. This finding is consistent with other studies, which showed that the distance between the anterior horn of the lateral ventricles and the IHF increased with fetal maturity and brain growth.8, 14 The pattern of the AW was comparable to the results of previous studies, which measured the AW of the fetuses in the axial plane and that of preterm neonates in the coronal plane from 24 to 42 weeks.8, 14 The AW was clearly seen in the early second trimester, decreased in the late second trimester, and relatively constant in the third trimester. Based on our observations and data from previous studies, the anterior horn of the lateral ventricle becomes smaller and straighter as GA increases.8, 19 As a result, examining and measuring the AW during the third trimester, when the calvarium has advanced ossification, is challenging.4 In contrast, Napolitano et al created reference curves using three-dimensional ultrasound volume acquisition with offline analysis, and showed that the AW increased with advanced GA.15 Moreover, in our study, the SD of the AW showed a narrower distribution with advanced GA. This finding is in contrast to Napolitano et al's study, which showed that the SD of the AW remained constant throughout pregnancy.15 Different acquisition and measurement techniques, and different maternal ethnicities, may possibly explain the discrepancy between the studies.20 In our study, the CSP width increased with gestational age, with a slight decrease at near term. This finding is consistent with previous studies.8, 16 Interestingly, using similar image acquisition and measurement techniques, the mean CSP width in our study is smaller than that in Falco et al's study.16 Effect of ethnicities may partly explain the difference in size of fetal brain structures.20 In brief, contradictory results among various studies possibly caused by racial factors, techniques, and types of ultrasound (2D/3D) as well as transducers imply that assessment of anterior complex should be based on the reference ranges derived from their own population and the same technique as well as equipment used in actual practice.
The VI and AW on the downside were slightly, but significantly, larger than those on the upside in the axial transventricular and coronal transcaudate planes. However, there was no difference in the VI or AW between the right and left sides of the fetal brain. These findings are consistent with those in a study by Ho et al.8 We hypothesize that the downside anterior horn is in a more dependent location, causing the minimal enlargement of this fluid-filled space. Some fetal CNS malformations, such as hemimegalencephaly, schizencephaly, and unilateral heterotopia, had an asymmetrical appearance and/or size. Therefore, fetal cerebral hemisphere symmetry cannot be assumed, and the proximal hemisphere should be considered in a brain morphological screening program in a low-risk population. Furthermore, examination of the fetal anterior complex should be carefully performed and measured separately in both sides. We have established reference ranges of downside and upside VI and AW in the axial and coronal planes, and these can be used in cases of suspected CNS malformation, especially asymmetrical malformations.
An advantage of this study is that the reference ranges of the VI were determined in both the standard axial transventricular and coronal transcaudate planes. A fetal brain examination by using multiple planes could be more beneficial for an accurate diagnosis.9 In addition, when the fetus is in a restricted position that limits achieving the standard axial plane, the coronal plane may be useful. However, in this study, the coronal plane was obtained by rotating the ultrasound probe 90° from the axial plane, and the fetal IHF was then perpendicular to the ultrasound beam. This coronal plane differed from the plane obtained in neonates in Brouwer et al's study, in which the IHF was parallel to the ultrasound beam.14 Therefore, in this study, reference values of the VI in the coronal plane may be different from neonatal values, and can be used specifically in the above-mentioned coronal plane of the fetus in utero.
Similar to previous studies,8, 15, 16 this study showed that transabdominal ultrasound successfully measured the VI, AW, and CSP width in more than 95% of cases. Moreover, the assessment was highly reproducible with excellent intra-/interobserver reliabilities, likely due to the use of standard planes and strict criteria for acquisitions and measurements. Therefore, we support incorporating the assessment of the anterior complex in standard transabdominal ultrasound examination of CNS because of its usefulness, feasibility, and reproducibility.
Strengths
The strengths are as follows: 1) We provide both models in predicting mean and those in predicting SD, enabling Z-score calculation for any actually measured values. 2) The models are highly reliable, based on the tests of goodness of fit, high reproducibility, supported by excellent agreement of measurements, and derived from low-risk pregnancy with postnatal confirmation of normality. 3) The reference ranges cover wide range of gestational age, enabling the use in both second and third trimester. 4) More options of reference ranges are provided, in terms of downside/upside hemisphere, standardized axial plane used in routine practice, and coronal plane added in targeted neurosonography. 5) Examples of clinical use contributing to early diagnosis of fetal CNS malformation cases are provided.
Limitations
This study has some limitations. First, our reference ranges were derived from Thai women. Therefore, tests for reproducibility when used in other populations may be required. Second, transvaginal ultrasound or a high-resolution linear or microconvex transducer, which might provide better image quality, was not used in this study. However, because of high success rate, high reproducibility, and higher acceptance in this study, transabdominal ultrasound may still be preferred in clinical practice. Third, 2% of fetuses were excluded since good quality images could not be obtained. However, because of this small number of exclusions, it is reasonable to conclude that measurement techniques are reproducible, consistent with that reported in a previous study.21 Finally, there was no long-term follow-up for infant outcomes, including neurodevelopment and behavioral abnormalities that may not be recognized at birth.20
Conclusion
Reference ranges of the anterior complex of the fetal brain, including downside and upside VI, downside and upside AW, and CSP width in the axial transventricular and coronal transcaudate planes, between 17 and 37 gestational weeks of low-risk singleton pregnancies have been constructed and available for clinical use.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




