Imaging of the Diaphragm Following Cardiac Surgery: Focus on Ultrasonographic Assessment
The authors have no conflicts of interest to report.
Abstract
Diaphragm dysfunction is a common complication following cardiac surgery. Its clinical impact is variable, ranging from the absence of symptoms to the acute respiratory failure. Post-operative diaphragm dysfunction may negatively affect patients' prognosis delaying the weaning from the mechanical ventilation (MV), extending the time of hospitalization and increasing mortality. Ultrasonography is a valid tool to evaluate diaphragmatic impairment in different settings, like the Intensive Care Unit, to predict successful weaning from the MV, and the Cardiovascular Rehabilitation Unit, to stratify patients in terms of risk of functional recovery failure. The aim of this review is to describe the pathophysiology of post-cardiac surgery diaphragm dysfunction, the techniques used for its diagnosis and the potential applications of diaphragm ultrasound.
Abbreviations
-
- CABG
-
- coronary artery bypass grafting
-
- COPD
-
- chronic obstructive pulmonary disease
-
- FRC
-
- functional residual capacity
-
- ICU
-
- intensive care unit
-
- MEP
-
- maximal expiratory pressure
-
- MIP
-
- maximal inspiratory pressure
-
- MV
-
- mechanical ventilation
-
- SBT
-
- spontaneous breathing trial
-
- SNIP
-
- sniff nasal inspiratory pressure
-
- TDI
-
- tissue Doppler imaging
-
- TF
-
- thickening fraction
-
- TLC
-
- total lung capacity
-
- US
-
- ultrasonography
-
- VC
-
- vital capacity
-
- VIDD
-
- ventilation-induced diaphragm dysfunction
The diaphragm is the most important inspiratory muscle, being responsible for 70% of the inspired air volume during regular breathing (Figure 1).1, 2 Patients undergoing cardiac surgery are at risk of diaphragmatic dysfunction which is often caused by phrenic nerve injury.3, 4 Postoperative diaphragm dysfunction may involve one or both the hemidiaphragms and it may be due to a partial or a complete loss of function (causing weakness or paralysis, respectively).5, 6 The incidence of diaphragm dysfunction after cardiac surgery ranges between 1 and 60%, but in some studies it reaches 75%.7, 8 This variability mainly depends on the differences in terms of surgical procedures and techniques used for its diagnosis.7
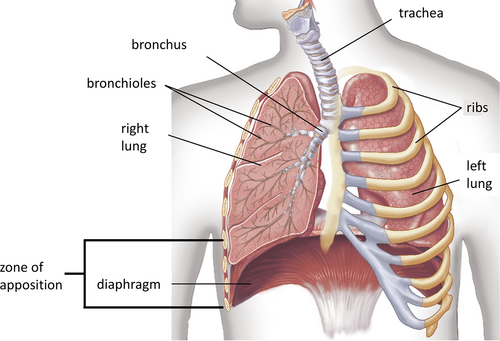
Many factors are related to the damage of the phrenic nerve during cardiac surgery, for example, the traumatic injury following the harvesting of the internal mammary artery (IMA) which is proximal to the nerve9 and the hypothermic demyelinating damage caused by the ice slush.10-12 Moreover, a prolonged time of curarization and/or intubation may induce diaphragmatic muscular atrophy leading to an increased risk of dysfunction.13 Eventually, sepsis, uncontrolled hyperglycemia, malnutrition, severe renal failure, and administration of neuromuscular blocking agents or high doses of corticosteroids may contribute to reduce diaphragmatic muscular strength, especially in mechanically ventilated patients.14
Diaphragm Dysfunction
Diaphragm dysfunction is a condition characterized by a reduced contractile function of the diaphragmatic muscle.12 It can be caused by muscular weakness, a partial loss of the contractile ability of the diaphragm, or muscular paralysis, the complete absence of the diaphragmatic capacity of contraction.4, 13 Weakness or paralysis can involve either one or both the hemidiaphragms. The type of respiratory failure possibly caused by diaphragm dysfunction is a “respiratory pump failure,” considering the “respiratory pump” as the total anatomical and functional apparatus that allows ventilation.14 The respiratory pump failure may produce an inadequate airflow due to a reduced respiratory effort to overcome increased resistance.14, 15 The alteration of ventilation–perfusion ratio provokes an increase of the physiological dead space, which is the part of the air volume that does not participate in gas exchange.16
Dysfunction may occur following a surgical procedure, in mechanically ventilated patients, in metabolic, inflammatory, or neuro-muscular disorders, in case of mediastinal or abdominal masses and diseases causing lung hyperinflation.12
Patients with unilateral diaphragmatic paralysis are usually asymptomatic at rest but may present exertional dyspnoea; patients with bilateral diaphragmatic dysfunction more frequently are symptomatic, even at rest.12, 17 Underlying cardiac diseases, obesity, or respiratory affections like chronic obstructive pulmonary disease (COPD) may exacerbate dyspnoea in diaphragm dysfunction, especially when patients are in supine position.12, 18
Patients with severe bilateral diaphragmatic dysfunction may present tachypnoea and anomalous activation of accessory respiratory muscles of ventilation at rest.19 The most typical sign of diaphragm paralysis is the “abdominal paradox” (also known as “thoraco-abdominal asynchrony”), consisting in the paradoxical inward motion of the abdomen during the inspiration.20
The evolution of the diaphragm dysfunction depends on its own cause. In degenerative neuromuscular diseases the course is progressive,12, 21-24 while in case of post-cardiac surgery only 2% of patients have persistent diaphragm paralysis at chest radiography after a 6-month follow-up.6
Assessment of Diaphragmatic Function
Several techniques can be used to assess diaphragmatic function.
The gold standard is the measurement of the negative pressure generated by diaphragm contraction in response to phrenic nerve stimulation (electrical or magnetic).25 During the stimulation, the difference between esophageal and gastric pressures (twitch transdiaphragmatic pressure, PDItw) can be evaluated using balloon catheters.25, 26 PDItw <15 cmH2O is suggestive of diaphragm dysfunction.25, 27, 28
At chest x-ray, the presence of an abnormally elevated hemidiaphragm (defined as the right hemidiaphragm located >2 cm higher than the left counterpart or the left hemidiaphragm placed at the same level or higher than the right one) is typical of unilateral diaphragm paralysis with a high sensibility (90%) but a low specificity (33%).29 False positives are attributable to congenital abnormalities of the diaphragm, hepatomegaly, distension of the splenic flexure of the colon, pulmonary atelectasis, and pleural effusion.29
Lung function tests may be performed to quantify the impact of diaphragmatic dysfunction on the ventilatory mechanics.25 Unilateral diaphragm weakness is typically associated with a mild decrease in vital capacity (VC) to about 75% of the predicted value, with a further 10–20% decrease in supine position; instead, functional residual capacity (FRC) and total lung capacity (TLC) are usually preserved in this situation.30, 31 In bilateral diaphragm weakness VC reaches ~50% of predicted value with a further decrease by 30–40% in supine position and reduced TLC with elevated residual volume can be found.16, 32 Pulmonary function testing can be used to measure the maximal inspiratory pressure (MIP) and the maximal sniff nasal inspiratory pressure (SNIP), which are indices of the respiratory muscles' strength.33-35 SNIP is measured at FRC using a pressure transducer connected to a catheter placed in the nostril.35 MIP and SNIP are reduced to about 60% of the predicted value in patients with unilateral diaphragmatic paralysis and to <30% in those with bilateral paralysis, while the maximal expiratory pressure (MEP) is usually preserved in patients with diseases that affect the diaphragm but spare the expiratory muscles.36
In the end, diaphragmatic ultrasonography (US) is a novel technique which can be adopted to assess both anatomical and functional characteristics of the diaphragm.
Diaphragm Ultrasonography
US is a non-invasive tool that can be rapidly performed at the bedside in different settings like the intensive care unit (ICU), the CS, and the CR Unit. It allows the visualization of structures below and above the diaphragm, possibly identifying extrinsic causes of pathological diaphragm elevation, such as abdominal masses and organomegaly.37
At US, the diaphragm is identifiable by its curved geometry and muscular echotexture.37 Longitudinally, the diaphragm is a hypoechoic structure enclosed by two highly hyperechoic structures which correspond to the pleura and the peritoneum (Figure 2).38 US mainly investigated the lateral and posterior portions of the diaphragm that represent the crural components of the muscle.37 These areas are more mobile than the central tendon region.39
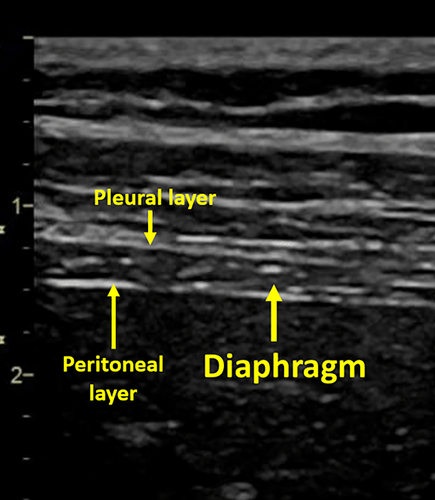
Diaphragm US is usually performed in the supine position due to a less side-to-side variability, and greater reproducibility.40, 41 Patients can be evaluated during quiet breathing, deep inspiration, or sniff maneuvers. Both hemidiaphragms can be examined with US. However, while the right hemidiaphragm is easily visualized through the liver window, the evaluation of the left hemidiaphragm is more complex due to the limited extension of the splenic window.40, 41 Ultrasonographic B-mode imaging allow the assessment of diaphragm structure and change in thickness during the respiratory cycle,42 while M-mode has a higher temporal resolution and it is useful to evaluate diaphragmatic excursion and contraction velocity.43
- Anterior subcostal view: it is the preferred view to measure diaphragmatic excursion. The anterior subcostal view is investigated using a low frequency transducer (2–6 MHz) placed in the anterior subcostal region between midclavicular and anterior axillary lines. The transducer is directed medially, cranially, and dorsally. B-mode imaging is adopted to assess diaphragm thickening, while M-mode is used to quantify the amplitude of the excursion pointing the ultrasound beam perpendicularly to the muscular hyperechoic zone. Amplitude and time of excursion can be used to calculate the diaphragm velocity of contraction.37
- Posterior subcostal view: it is evaluated with the patient in the sitting position collocating a low-frequency transducer in the posterior subcostal region. This view allows to assess the same parameters which are obtainable through the anterior subcostal view.37
- Intercostal view: it is assessed positioning a high-frequency transducer (7–18 MHz) at the intercostal space between the seventh and eighth or eighth and ninth ribs along the anterior axillary line.37 This view is good for measuring diaphragmatic thickness in both B-mode and M-mode.
- Subxiphoid view: the patient should be in the supine position. A low-frequency transducer is placed horizontally under the xiphoid process. The probe is oriented upwards obtaining a 45° angle with the anterior abdominal wall. Using the B mode, both hemidomes can be seen at the same time on an oblique transverse view, and a qualitative comparison of ultrasound parameters can be done in real-time.44 This view is useful to evaluate diaphragm excursion, time of excursion and velocity of contraction.
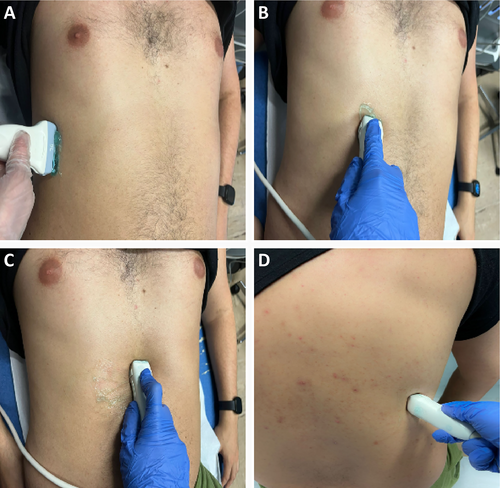
- Diaphragm thickness: it is assessed with the intercostal approach either in B-mode or M-mode visualizing the so-called “zone of apposition” which is the portion of the diaphragm leaning on the inferior region of the ribcage.21, 45 Thickness measured by US has been shown to correlate with direct diaphragm thickness measurements on cadavers.46 An end-expiratory thickness <2 mm is considered as an ultrasonographic criterium of diaphragmatic atrophy.15 The value of thickness is influenced by the intercostal space where the probe is positioned as the inferior portions of the diaphragm are thicker than the superior ones.37
- Thickening fraction (TF): it represents the change of diaphragmatic thickness during inspiration. Fractional change in diaphragmatic thickening is calculated with the following formula: TF = (thickness at end-inspiration − thickness at end-expiration)/thickness at end-expiration.47 Physiological TF is a reproducible parameter which ranges between 28 and 96% and reduces to −35 to 5% in case of diaphragmatic paralysis.48 A value below 20% is considered as a marker of diaphragm paralysis.35
- Diaphragm excursion: on M-mode, the diaphragm appears as a single thick hyperechoic line, and its movements during the respiratory cycle can be analyzed with a high temporal resolution.37, 49 The excursion is the overall movement that the diaphragm performs from the beginning of the respiratory cycle to the inspiratory peak. Lerolle et al proposed a diaphragm excursion >2.5 cm as cut-off to exclude severe dysfunction in patients following cardiac surgery,50 while 1.8 cm corresponded to the lower limit of normal in a study performed on 210 healthy subjects by Boussuges et al.43 During quiet breathing, diaphragm weakness is defined as excursion <1 or 1.5 cm.51, 52 Diaphragm excursion during deep breathing in healthy patients is typically asymmetrical, with higher amplitude on the left side.37 Side-to-side variability, defined as the right-to-left ratio of maximal excursion, ranges from 0.5 to 2.5 in quiet breathing and from 0.5 to 1.6 in deep inspiration.43, 53
- Velocity of contraction: it corresponds to the ratio of excursion over time of inspiration.6 It can be evaluated during quiet breathing, deep inspiration, or sniff maneuvers. The velocity of diaphragm contraction has been proved to rise almost 7-fold from 1.52 cm/s (during quiet breathing) to 10.4 cm/s during sniff.54
- Peak contraction velocity, peak relaxation velocity, and maximal relaxation rate: reproducible tissue Doppler imaging (TDI) parameters assessed using a low-frequency transducer. These measurements resulted lower in critically ill patients in comparison to healthy subjects, despite there are no specific cut-offs for diaphragm dysfunction.55 In the same population, peak contraction velocity seems to be strongly correlated with peak transdiaphragmatic pressure and pressure–time product, whilst the transdiaphragmatic pressure-derived maximal relaxation rate was significantly correlated with TDI-maximal relaxation rate.55
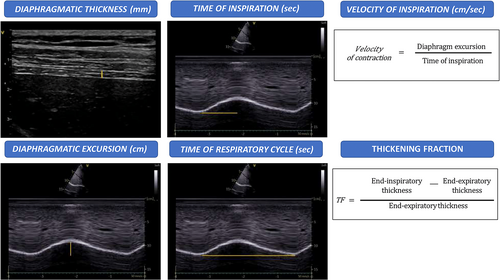
Table 1 shows the lower limit of normal of the main US diaphragmatic parameters.
Post-Cardiac Surgery Diaphragm Dysfunction and the Role of Ultrasound Assessment
Surgical injuries represent one of the major causes of unilateral diaphragm dysfunction. Tralhão et al described that cardiac surgery procedures are usually followed by a transient phase of diaphragm functional impairment called “diaphragmatic stunning,” which is characterized by a reduced amplitude of excursion. The stunning starts 48 hours after surgery and lasts, on average, until the fifth post-operative day.59 In the 36% of cases, the transient reduction of the excursion was severe enough to be graded as “dysfunctional,” potentially leading to prolonged MV and other pulmonary complications.59 The overall incidence of post-cardiac surgery diaphragm dysfunction is not clear as it depends on the surgical procedure characteristics and the techniques used for its detection.60 Coronary artery bypass grafting (CABG) is a technique that may be associated with a traumatic injury of the left phenic nerve due to the anatomical proximity to the left IMA. The incidence of post-CABG diaphragm dysfunction ranges between 1 and 60%.7 This enormous variability is partially justified by the different existing surgical approaches used to perform the grafting.
Even if some studies have identified CABG as a predictor of post-operative diaphragm dysfunction,4 our group recently found that diaphragm dysfunction was even more frequent in patients undergoing other-than-CABG cardiac surgery procedures compared with the surgical revascularization (79.1 vs 46.7%).6 This suggests that there are mechanisms causing post-cardiac surgery diaphragm dysfunction other than the phrenic nerve damage during the harvesting of the left IMA. The cardiac cooling with topical ice slush may induce post-operative diaphragm dysfunction due to the demyelinating injury caused by the exposure to low temperatures.61 The avoidance of hypothermic injury with the use of cardiac insulation pads and the replacement of topical ice slush with cardioplegia solutions significantly decrease the risk of diaphragmatic dysfunction.62
Trying to reduce the factors favoring post-surgical diaphragm dysfunction is fundamental as it negatively affects patients' prognosis with an increased risk of longer ICU stay, longer time of hospitalization and post-operative pneumonia.4, 63 Post-cardiac surgery diaphragmatic dysfunction may also impact on the postoperative rehabilitative period as the affected patients frequently need tailored programs of respiratory exercises to improve their clinical status.6 Arterial hypertension and obesity procedures were identified as main predictors of post-operative diaphragm dysfunction.4
Another determiner of post-cardiac surgery dysfunction is the MV.64 Ventilation-induced diaphragm dysfunction (VIDD) is reported in up to 53% of mechanically ventilated patients within 24 hours of intubation and an additional 26% may develop VIDD while on mechanical ventilation during their stay in the ICU.65 The pathophysiological feature of the VIDD is the disuse atrophy which is associated with increased oxidative stress, downregulation of protein synthesis, and activation of proteolytic pathways inside the muscle cells.9 Moreover, ultrasonographic assessment of diaphragmatic function has been shown to predict the failure of weaning from MV,53, 66 boosting the interest for this technique in the ICU in the last years. In a population of mechanically ventilated patients in the Intensive Care Unit, Spadaro et al described a new index, the diaphragmatic-Rapid Swallow Breathing Index (dRSBI = respiratory rate/diaphragm excursion), which resulted more accurate than traditional RSBI (respiratory rate/tidal volume) in predicting the weaning outcome following the T-tube spontaneous breathing trial (SBT).67
One of the main limitations of diaphragmatic US is the lack of pathological cut-offs in specific conditions like the post-cardiac surgery context. Although no specific cut offs have been established for post-operative cardiac patients, the most reliable parameter to assess diaphragm function seems to be the excursion, with the value of 2 cm as a valid cut-off to define diaphragm dysfunction.6, 59 Some other authors, instead, adopted TF <20% as diagnostic criterion for this condition.8 However, although TF seems to be as accurate as excursion to define diaphragmatic functional impairment, it is also a less feasible and reproducible measurement.45 The other US parameters (velocity and respiratory times) show more physiological variations according to age, gender, and health status of the patient: they are more useful for a multiparametric assessment rather than as unique elements to diagnose diaphragm dysfunction.6
Diaphragm US may be a useful tool to assess patients' clinical course even in the setting of CR. The incidence of diaphragmatic dysfunction, evaluated as US excursion <2 cm, is about 70% in CR following cardiac surgery.6 This condition has a negative impact on the post-operative functional recovery as patients with persistent diaphragm dysfunction after a 10-session rehabilitation program show lower level of functional performance at the 6-minute walking test (6MWT).6 Combined surgical procedures and post-operative pneumothorax are strong predictors of failure of diaphragmatic improvement during CR.6
Conclusion
Diaphragm dysfunction is a frequent complication after cardiac surgery which negatively affects patients' clinical outcomes. Several techniques can be used to diagnose diaphragm dysfunction. US is an accurate, non-invasive tool to identify and monitor post-operative diaphragm dysfunction in different settings like the CS and the CR Unit. Diaphragm US parameters are also useful to optimize the ventilatory weaning among the ICU patients. Further studies are necessary to establish definite US cut-offs for diaphragm dysfunction in specific populations and to define standardized protocols with the use of diaphragm US in the clinical practice.
Acknowledgment
Open access funding provided by BIBLIOSAN.
Open Research
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




