Phase 1 evaluation of an elastomeric nucleus pulposus device as an option to augment disc at microdiscectomy: Experimental results from biomechanical and biocompatibility testing and first in human
Abstract
Objective
Whilst microdiscectomy is an excellent reliever of pain for recalcitrant lumbar disc herniation (LDH), it has a high failure rate over time due to the ensuing reduction in mechanical stabilization and support of the spine. One option is to clear the disc and replace it with a nonhygroscopic elastomer. Here, we present the evaluation of biomechanical and biological behavior of a novel elastomeric nucleus device (Kunovus disc device [KDD]), consisting of a silicone jacket and a two-part in situ curing silicone polymer filler.
Materials and Methods
ISO 10993 and American Society for Testing and Materials (ASTM) standards were used to evaluate the biocompatibility and mechanics of KDD. Sensitization, intracutaneous reactivity, acute systemic toxicity, genotoxicity, muscle implantation study, direct contact matrix toxicity assay, and cell growth inhibition assay were performed. Fatigue test, static compression creep testing, expulsion testing, swell testing, shock testing, and aged fatigue testing were conducted to characterize the mechanical and wear behavior of the device. Cadaveric studies to develop a surgical manual and evaluate feasibility were conducted. Finally, a first-in-human implantation was conducted to complete the proof of principle.
Results
The KDD demonstrated exceptional biocompatibility and biodurability. Mechanical tests showed no Barium-containing particles in fatigue test, no fracture of nucleus in static compression creep testing, no extrusion and swelling, and no material failure in shock and aged fatigue testing. Cadaver training sessions showed that KDD was deemed implantable during microdiscectomy procedures in a minimally invasive manner. Following IRB approval, the first implantation in a human showed no intraoperative vascular and neurological complications and demonstrated feasibility. This successfully completed Phase 1 development of the device.
Conclusion
The elastomeric nucleus device may mimic native disc behavior in mechanical tests, offering an effective way for treating LDH by way of Phase 2 and subsequent clinical trials or postmarket surveillance in the future.
1 INTRODUCTION
The conventional invasive surgical approach, microdiscectomy, is performed for recalcitrant sciatica as a consequence of sequestrated lumbar disc herniation (LDH). Microdiscectomy is the most common spinal surgery performed worldwide, with nearly 500 000 performed annually in the USA alone and 12 000 performed in Australia every year. During the microdiscectomy surgery, either the loose fragment in the canal is removed (fragmentectomy) or complete clearance of the nuclear cavity is performed with no prosthetic replacement offered. Although microdiscectomy is widely perceived as a successful procedure for immediate pain relief, it has a high failure rate over time due to the ensuing reduction in mechanical stabilization and support of the spine. Clinical data have shown that nearly a third of discectomy patients are dissatisfied with their surgical outcomes at 12-month follow-up.1 Furthermore, one in five patients will undergo a repeat surgery within the first 7 years of their microdiscectomy surgery.2, 3 Therefore, researchers have an increasing interest in nucleus pulposus (NP) substitutes that aim to mimic the native NP biologically as well as mechanically.4, 5
Over the past decades, several constituents have developed different designs and biomaterials for nucleus augmentation devices.6-8 Based on the design principles and materials used, nucleus augmentation devices are divided into preformed mechanical devices such as NuBac (Pioneer Surgical Technology, Michigan) and DIVA® (SC Medica, Strasbourg, France), a preformed elastomer such as PDN (Raymedica, Minnesota), and in situ curing devices such as DASCOR (Disc Dynamics, Minnesota), NuCore (Spine Wave, Connecticut), Percutaneous Nucleus Replacement (PNR; TranS1, Colorado), and PerQdisc (Spinal Stabilization Technologies, Ireland). The aim of nucleus augmentation device is to reconstruct the NP primarily while maintaining the biomechanics of the annulus fibrosus (AF) and vertebral endplates, which are theoretically designed to provide stable motion, increase intervertebral disc (IVD) height, relieve, or lessen transmission of shear forces on the remaining AF, and stabilize spinal ligamentous structures.9 Clinically, a nucleus augmentation device is mainly designed to treat patients with discogenic pain caused by degenerative disc disease and/or neurogenic pain caused by disc herniation. However, past attempts have not been successful due to inadequate delivery systems or noncontainment of curing material of previous devices. In the meanwhile, their application in clinical practice has remained elusive.10
As per literature, a functional nucleus augmentation device must meet five essential criteria11: (1) biocompatible and durable to survive the lifespan of the recipient; (2) stabilize motions across all axes of movement, and restore normal distribution of loads in the motion segment; (3) high conformity in the nucleotomized cavity to avoid device migration and subsidence; (4) optimal stiffness to avoid excessive wear and/or remodeling of the endplate; and (5) easy to implant and remove, minimize any additional damage to the AF during implantation.
Based on the essential design criteria, polymeric hydrogel is one of the promising materials for nucleus augmentation device.12 Polymeric hydrogels, such as polyethylene, polyvinyl alcohol, silicones, polyurethanes, and polymers reinforced with fibers or ceramics, have been investigated as suitable augmentation materials for NP.12, 13 Despite the intensive research over the past decade about these polymers for nucleus augmentation devices, none yet exhibit characteristics like the natural IVD in terms of biomechanics behavior, adequate stiffness, and cellular responses between the implant and biological environment.14
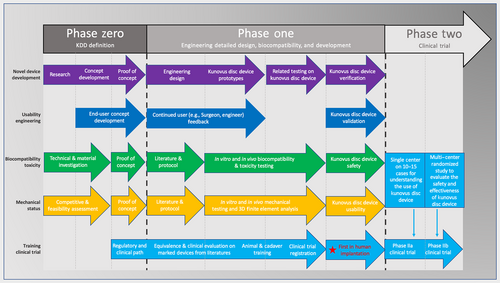
In this direction, our group developed an injectable in situ curing elastomeric device (Kunovus disc device [KDD], Kunovus Technologies) that uses a biomaterial that has a long history of human implantation and has a stiffness characteristic of a young person's nucleus. The material is contained within a silicone jacket that conforms to the shape of the nucleotomised cavity when inflated and uses a proprietary system of sophisticated delivery instruments to fill the jacket with an inert in situ curing elastomeric filler material. Finite element analysis study and in vitro mechanical testing supported that KDD could restore the axial compressive mechanical properties of a disc after a discectomy and the kinematic changes of a degenerated disc represented by the nucleotomised single motion segment.15, 16 However, to our knowledge, there are no related studies assessing the silicone made in situ curing elastomeric nucleus augmentation device (Table 1). In this study, we evaluate the biomechanical and biocompatible properties of the novel nucleus augmentation implant (KDD). In order to assess the surgical feasibility of implanting the in situ curing elastomeric nucleus augmentation device, we implanted the device in human cadaveric lumbar spine following discectomy. Finally, a first-in-human implantation demonstrating clinical feasibility was conducted to complete Phase I of the device development (Figure 1).
| Features | Preformed mechanical devices | Preformed elastomer | In situ curing devices | |||||
|---|---|---|---|---|---|---|---|---|
| NuBac (Pioneer Surgical Technology, Michigan) | DIVA® (SC Medica, Strasbourg, France) | PDN (Raymedica, Minnesota) | DASCOR (Disc Dynamics, Minnesota) | NuCore (Spine Wave, Connecticut) | Percutaneous Nucleus Replacement (PNR; TranS1, Colorado) | PerQdisc (Spinal Stabilization Technologies, Ireland) | Kunovus disc device | |
| Material | PEEK | Titanium | Hydrogel pellet in PE jacket | Polyurethane | Silk and elastin | Silicone | Silicone | Silicone |
| Size and diameter | Fixed | Fixed | Fixed | Fixed | Fixed | Fixed | Based on the measurement | Based on the measurement |
| Mechanical and wearing behavior | In vitro test, high contact stress to endplate | Not mention | In vitro test, potential extrusion or expulsion | In vitro test, potential extrusion or expulsion | In vitro test, potential extrusion or expulsion | In vitro test, potential extrusion or expulsion | In vitro test + finite element study | In vitro test + finite element study |
| Biocompatibility | Not mention using animal model | Not mention using animal model | Not mention using animal model | Not mention using animal model | Not mention using animal model | Not mention using animal model | Not mention using animal model | Test using animal model |
| Conformity | High conformity, safety device | High conformity, safety device | High conformity, safety device | High conformity, safety device | High conformity, safety device | High conformity, safety device | High conformity, safety device | High conformity, safety device |
| Ease of implant/removal | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Reoperation rates | >3 years: 52.6%38 | 2 years: 4.5%7 | >3 years: 27.1%38 | 1–3 years: 10%34 | 1–3 years: 035 | >3 years: 57.7%38 | Not mention | <1 year: 0 |
2 MATERIALS AND METHODS
2.1 KDD characteristics
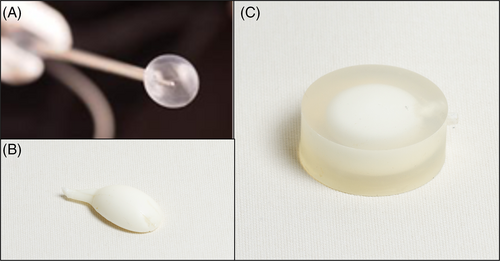
The KDD consists of a silicone jacket (Figure 2A) and a two-part in situ curing silicone polymer filler (Figure 2B). The silicone jacket is molded from a two-part translucent liquid silicone rubber typically used for injection molding. The silicone filler is two-part liquid silicone rubber, each filled separately into one of the two barrels of the filler assembly. Silicone rubber polymerization is initiated when the two liquid silicone polymers are mixed in the filler tube assembly prior to injection into the jacket. The material then cures (hardens) in situ by the formation of cross-linkages to create the solid silicone elastomer. The silicone filler contains an additive Barium sulfate that provides a radiopaque feature to the implant. The implant provides a one-size-fits-all prosthesis that deforms elastically to completely conform to the disc cavity following the nucleotomy. Once dispensed into the jacket, the filler material (silicone polymer) cures within ~8–10 min, forming the implant. A sophisticated delivery system allows for sizing, deep placement under fluoroscopy guidance, and eventual detachment from the delivery system of the KDD as it cures.
2.2 Biocompatibility tests
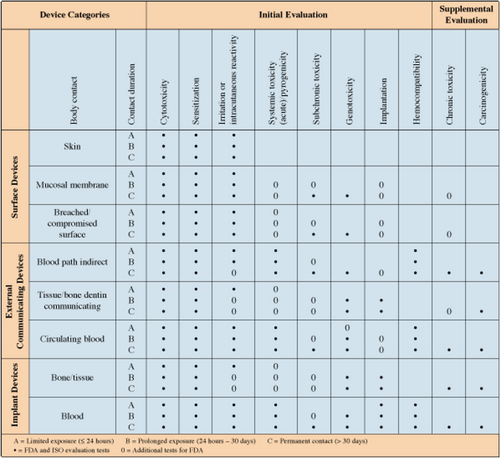
Independent laboratories have performed biocompatibility testing (in vivo and in vitro test) on KDD, in accordance with the relevant parts of the following guidelines/standards: tests for cytotoxicity (International Organization for Standardization ISO 10993-5), tests for irritation and delayed-type hypersensitivity (ISO 10993-10), tests for system toxicity (ISO 10993-11), tests for genotoxicity, carcinogenicity, and reproductive toxicity (ISO 10993-3), tests for local effects after implantation (ISO 10993-6), and general requirements for the competence of test and calibration laboratories (ISO 17025; Figure A1). Additional biocompatibility and safety studies have been performed, including evaluation of cytotoxicity using the direct contact material toxicity assay, evaluation of cytotoxic potential using a cell growth inhibition assay, sterility evaluation assay-microbial support, sterility evaluation by direct inoculation, and testing for implant chemical extractables and degradation products (Supporting Information S1).
2.2.1 Tests for cytotoxicity
A single extract of the test article was prepared using single-strength Minimum Essential Medium supplemented with 5% serum and 2% antibiotics (IX MEM). This test extract was placed onto three separate monolayers of L-929 mouse fibroblast cells propagated in 5% CO2. Three separate mono layers were prepared for the reagent control, negative control, and for positive control. All monolayers were incubated at 37°C in the presence of 5% CO2, for 48 h. The monolayer in the test, reagent control, negative control, and positive control wells was examined microscopically at 48 h to determine any change in cell morphology.
2.2.2 Tests for irritation and delayed-type hypersensitivity
The test article was extracted in 0.9% sodium chloride USP (SC) and sesame oil, NF (SO). Each extract was intradermally injected and conclusively patched to 10 test guinea pigs (per extract) in an attempt to induce sensitization. The vehicle was similarly injected and conclusively patched to five control guinea pigs (per vehicle). Following a recovery period, the test and control animals received a challenging patch of the appropriate test article extract and the reagent control. All sites were scored 24 and 48 h after patch removal.
A 0.2 mL dose of the appropriate test article extract was injected by the intracutaneous route into five separate sites on the back of each rabbit. Similarly, the corresponding control was injected into the back of each rabbit. The injection sites were observed immediately after injection. Observations for erythema and edema were conducted at 24, 48, and 72 h after injection.
2.2.3 Tests for systemic toxicity
A single dose of the appropriate test article extract was injected into each of five mice per extract by either the intravenous or intraperitoneal route. Similarly, five mice were dosed with each corresponding blank vehicle. The animals were observed immediately and at 4, 24, 48, and 72 h after systemic injection.
2.2.4 Tests for genotoxicity, carcinogenicity, and reproductive toxicity
The saline and dimethyl sulfoxide (DMSO) test article extract was found to be noninhibitory to the growth of tester strains TA98, TA100, TA1535, TA1537, and WP2uvrA. Separate tubes containing 2 mL of molten top agar supplemented with histidine-biotin solution for the Salmonella typhimurium strains and with tryptophan for the Escherichia coli strain were inoculated with 0.1 mL of culture for each of five tester strains, and 0.1 mL of the DMSO extract. A 0.5 mL aliquot of sterile Water for Injection or 59 homogenates, simulating metabolic activation, was added when necessary.
The mouse lymphoma L5 I 78Y/K+/− cell line, heterozygous at the thymidine kinase locus on chromosome 11 was used for this assay (Mouse Lymphoma Assay). For 3 consecutive days (Days 1, 2, and 3), 12 mice per test article extract (6 per sex) were injected intraperitoneally with the test article extracts (Mouse Peripheral Blood Micronucleus Study). Similarly, 12 mice per extract vehicle were dosed with the appropriate vehicle as the negative control condition and 12 mice were dosed with the positive control, Methyl methanesulfonate. All animals were observed immediately following injection and daily for general health. On Day 4, blood was collected from the tail veins and solutions were prepared.
2.2.5 Tests for local effects after implantation
Sterile implant samples were prepared aseptically. Negative control samples were sterilized by steam. Rabbits were implanted and were then euthanized 2 weeks later. Muscle tissues were excised, and the implant sites were examined macroscopically. A microscopic evaluation of representative implant sites from each rabbit was conducted to further define any tissue response.
Muscle implantation was conducted to determine the potential for irritation or toxicity of the KDD in rats at 2 and 12 weeks.
2.3 Biomechanical tests
2.3.1 Standards/guidelines for tests
The following standards were used for reference and for preparing specific test protocols: American Society for Testing and Materials (ASTM)-F2346-05, ASTM-WK 4863, ASTM-F1877-98, ASTM-F2423-05, ASTM-F2789-10, and ISO/DIS-18192-1.
2.3.2 Test methods
A surrogate annulus model, see Figure 2C, was made of silicon (Silicone Shore Hardness 60A). The stiffness of the silicone can produce an appropriate load response due to the appropriate ratio of base and curing agent. The geometry of the annulus model was matched in overall height, shape, and wall thickness variation to anatomic data. This version is a simple monolithic material without layering or internal fiber reinforcement.
The surrogate annulus model is unique in that the mechanical properties of each component of the disc can be easily and quantifiably altered to match the desired physiological responses of the spinal motion segment, providing a simple and accessible means of testing NP replacement materials, examining the effects of age-related degeneration on AF properties, and exploring characteristics of annular defects and mechanisms of nucleus extrusion.
The KDD was implanted into the cavity of the surrogate annulus model on Perspex constraining plates. The silicone filler was injected into the cavity until the KDD completely filled the cavity of the annulus model. A range of tests was conducted to characterize the mechanical and wear behavior of the implant, including fatigue testing, wear testing, static compression testing, expulsion testing, swell testing, shock testing, and aged fatigue testing. It examined the following performance characteristics of the KDD: disc height change, range of motion (ROM), and stiffness (motion resistance).
2.3.3 Fatigue testing
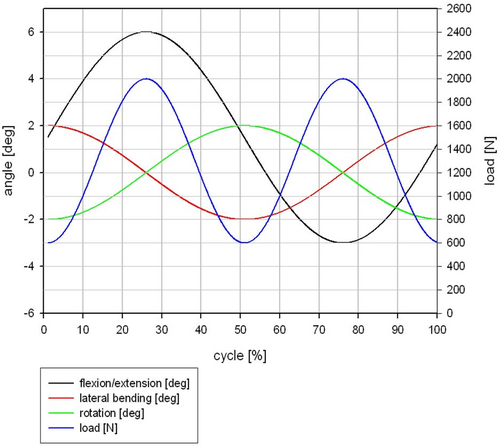
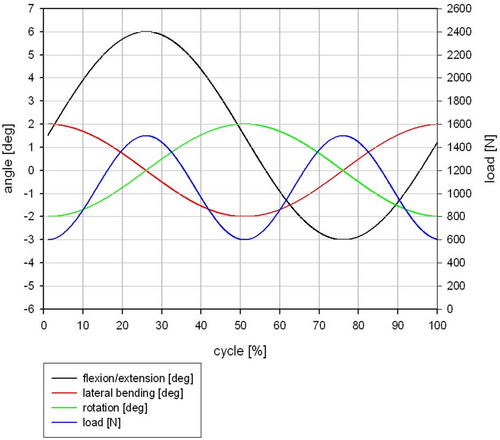
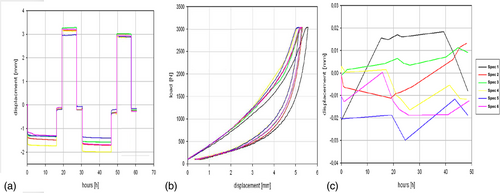
The fatigue test was done under simulated in vivo loading conditions over 10 million cycles in compression and 5 million cycles in flexion/extension, lateral bending, and axial rotation based on ISO/FDIS 18192-1 with EndoLab® six station spine simulator (EndoLab Mechanical Engineering GmbH, Thansau, Germany; Figure 3A). A total of six specimens were tested by EndoLab Mechanical Engineering GmbH (Germany) under two different loads (Figures A2 and A3) in sinusoidal dynamic fatigue model at 2 Hz to establish the device's fatigue strength. An optical microscope was used to examine the surface of the KDD for microparticle formation, surface roughness, crack propagation, and abrasion. Specimens were kept immersed in calf serum during fatigue testing. Both the immersion fluid and test article wash fluids were collected at the end of testing for particle analysis.
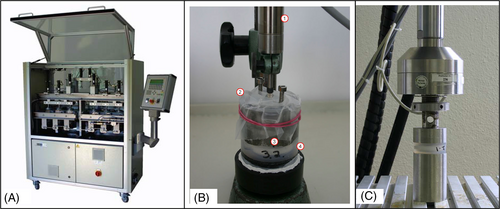
2.3.4 Wear testing
Wear tests were conducted to gain basic information about the particles generated during fatigue tests. Hydrochloric acid has been used to devoid biological particles (e.g., native proteins, partially denatured proteins, and corrosion).17 When the shape of particles has been confirmed by Analysis Pro von Soft Imaging System GmbH, the energy dispersive x-ray (EDX) analysis was used to distinguish whether the particles come from the nucleus implant or the annulus fixation.
2.3.5 Static creep testing
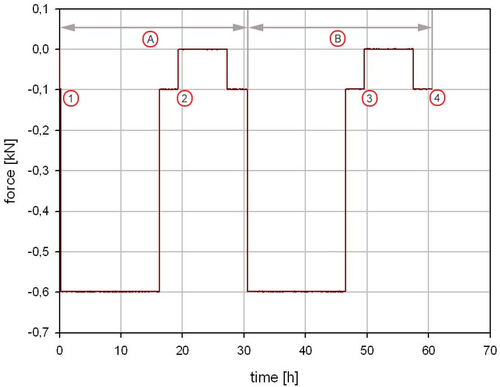
Static creep testing was performed to characterize the creep and recovery profile of the KDD nucleus implant under a constant load. Test conditions replicated forces exerted on the IVD when a person stands continuously with an axial load from 100 to 600 N for periods of 16 h followed by 8 h of rest, over 48 h (Figure A4).
2.3.6 Expulsion testing
Expulsion testing was conducted on partially filled nucleus implants in the surrogate annulus model to evaluate the risk of nucleus extrusion. A total of six specimens were tested with 100 000 cycles at 2 Hz with the compressive load ranging between 600 and 2000 N and with 2000 N load in flexion and extension (from −6° to 3°) at 1 Hz.
2.3.7 Swell testing
The specimens (n = 6) have been dried in an oven at 100°C for at least 4 h before the swell test. Afterward, specimens have been placed in the test station for 48 h as shown in Figure 3B. The test station has been filled with ringer solution at ambient room temperature. An elastic foil was covered on the specimens for preventing evaporation. The axial change in height has been measured at least once every 24 h with a deflection sensor (Megatron, EDCT20-S-2410, serial number 35597). The deflection sensor has been calibrated by the means of gauge blocks (Endolab intern: PM144).
2.3.8 Shock testing
IVDs in the lumbar spine may be subjected to supra-physiological loads during trauma incidents. The performance of the implant (n = 6) has been evaluated under extreme loading conditions (Figure 3C). Compressive force cycles of magnitude up to 3 kN were applied at a rate of 200 kN/min to the KDD, contained inside the surrogate annulus model.
2.3.9 Aged fatigue testing
The KDD specimens were treated in a dry oven and water bath to age the implants by an equivalent of 24 years. The aged samples were subjected to test conditions previously described under fatigue testing.
2.4 Testing of silicone filler for estimation of shelf life
The device design requirements specify that the ready-to-use liquid silicone rubber polymers developed as two-part silicone elastomer to fill the KDD implant jacket should have at least 6 months of shelf life after sterilization. The silicone elastomer filler in the Filler Barrel Assembly (FBA) was tested after 6 months of ambient storage to verify that the combined material using the static mixer meets the design requirements for Scorch Time and T90.
2.5 Thermodegradation testing of silicone filler
Three silicone specimens were tested by thermal gravimetric analysis. The percentage weight loss was evaluated at 750°C and 400°C.
2.6 Cadaver training
Three human cadaver training sessions were conducted by the surgeons (Saeed Kohan, Alisha Sial, and Ashish D. Diwan) at Macquarie University (Sydney, Australia) on donated human bodies under ethics approval to assess the safe implantability of the KDD and to perform an assessment of its human-interface engineering (Supporting Information S2). The surgical procedure was deemed successful if: (1) the disc space was able to be adequately cleared using the selected tools; (2) the Kunovus system was able to measure the volume of the disc cavity following the microdiscectomy; (3) the KDD was implanted at the desired level using the Kunovus System; (4) the surgeon was able to conduct the entire procedure with the guidance available in the current surgical manual; (5) the KDD showed no signs of leakage after the removal of all instruments; and (6) the Kunovus System was able to dispense the implant material without seizing or excess movement of the instruments within the cadaver. During cadaver training sessions, the overall verification of the Kunovus System and surgical technique for implantation of the KDD was evaluated for readiness for clinical use.
2.7 Clinical study
After extensive preclinical testing, a clinical study was initiated with a 12-month follow-up (Supporting Information S3). The KDD was indicated for the treatment of adult patients with one level of primary or recurrent LDH in the lumbar spinal region L3 to S1. The consented patient was a suitable candidate for the treatment of the LDH via a microdiscectomy procedure with unrelieved back and /or leg pain due to damaged spinal discs and whose symptoms had not improved over the last 6 months with conservative treatment. The overall objective of this trial is to evaluate the safety and effectiveness of the KDD in maintaining lumbar disc height in subjects undergoing single-level microdiscectomy for LDH. Disc height of surgical level (disc height is expressed as average of the anterior, middle, and posterior IVD height, and disc height index [DHI] is expressed as a ratio of the sum of anterior and posterior IVD height to the sum of superior and inferior disc depth), Oswestry Disability Index (ODI), and Numeric Pain Rating Scale (NRS) were collected preoperatively and 6 weeks, 1, 3, 6, and 12 months postoperatively.
For the purposes of this article, the first study case of the above clinical trial is reported as it completes Phase 1 (Real world Feasibility) of device development.
2.8 Statistical analysis
Continuous data are presented as mean ± SD. Dichotomous data are presented as numbers and percentages.
3 RESULTS
3.1 Biocompatibility testing results
All the results have been listed in Table 2. The KDD test article was not cytotoxic and showed no evidence of causing cell lysis or toxicity. None of the animals responded to the topical challenge. The KDD did not cause delayed contact sensitisation in guinea pigs and did not cause mortality or systematic toxicity in the mice. Intracutaneous injections of KDD test extracts into Rabbits were administered to determine no erythema and edema local dermal irritant effects. The KDD was nonmutagenic to bacteria and mouse lymphoma cells, and not genotoxic to the mouse. All test sites of rabbit muscle tissue at 2 and 12 weeks, when viewed at low magnification showed no evidence of capsule formation or adverse reaction. Microscopic evaluation of tissue response indicated that the KDD was nonirritant. The additional studies showed that the elastomer materials were not cytotoxic and not affectionate in cell growth.
| Purpose (study description) | Test Article: Kunovus disc device (KDD) | Test and results summary |
|---|---|---|
| Test and control articles | ||
Cytotoxicity Cytotoxicity study using the ISO elution method |
Test vehicle: 1× Minimum Essential Medium (MEM) extract of KDD Reagent control: 1× MEM extract without test material Negative control: High-density polyethylene (HDPE) Positive control: Tin stabilized polyvinylchloride |
In vitro biocompatibility study, based on ISO 10993 Part 5: Tests for cytotoxicity, was conducted on the KDD with mouse fibroblasts to determine the potential for cytotoxicity The KDD test article was not cytotoxic and showed no evidence of causing cell lysis or toxicity after 48 h. The reactivity of the positive control was severe. The negative control was not reactive |
Sensitization ISO maximization sensitization—extract |
Test vehicles: 0.9% sodium chloride USP solution (SC) and sesame oil, NF (SO) Control: Vehicle without test material |
Guinea pig maximization test of the KDD, based on the requirements of ISO 10993 Part 10: Tests for Irritation and Delayed-type hypersensitivity was conducted to evaluate the potential for delayed dermal contact sensitisation At 24 and 48 h, no visible change was observed at all test and control sites. None of the animals responded to the topical challenge. The KDD did not cause delayed contact sensitisation in the animal |
Intracutaneous reactivity ISO intracutaneous study—extract |
Test vehicle: 0.9% sodium chloride USP solution (SC) and sesame oil, NF (SO) 0.9% sodium chloride USP solution (SC) and sesame oil, NF (SO) Control: Vehicle without test material |
The KDD was evaluated for intracutaneous reactivity in rabbits, based on the requirements of ISO 10993 Part 10: tests for irritation and delayed-type hypersensitivity. intracutaneous injections of KDD test extracts were administered to determine the potential for local dermal irritant effects At 24, 48 and 72 h after injection, there was no erythema and no edema from the saline KDD extract. There was very slight to well-defined erythema and very slight edema from the sesame oil KDD extract. There was no difference in mean reaction scores between the KDD test article and control |
Acute systemic toxicity ISO systemic toxicity study—extract |
Test vehicle: 0.9% sodium chloride USP solution (SC) and sesame oil, NF (SO) Control: Vehicle without test material |
KDD extracts were evaluated for systemic toxicity, in accordance with ISO 10993 Part 11: Tests for systemic toxicity guidelines, through intravenous or intraperitoneal injections in mice Animals appeared clinically normal and gained weight throughout the study. There was no difference between mice treated with KDD extract and the corresponding control. The KDD did not cause mortality or systemic toxicity in the mice |
Genotoxicity Bacterial reverse mutation study—extract |
Test vehicles: 0.9% sodium chloride USP solution (SC) and dimethyl sulfoxide (DMSO) Negative control: Vehicle without test material Positive control: Dexon paradimethylaminobenzene diazosulfonic acid sodium salt |
Bacterial Reverse Mutation was conducted to satisfy, in part, the genotoxicity requirement of ISO 10993 Part 3: Tests for Genotoxicity, Carcinogenicity and Reproductive Toxicity At 48 h, Salmonella typhimurium and Escherichia coli strains exhibited appropriate genetic characteristics and no significant spot plate inhibition was observed. The saline and DMSO KDD extracts did not cause a >2-fold increase in mean number of revertant of tester strains. The KDD was nonmutagenic to bacteria |
Genotoxicity Mouse lymphoma assay—extract |
Test vehicles: 0.9% Sodium chloride injection, USP (SC) and DMSO Negative control: Vehicle without test material Positive controls: Methylmethane sulfonate (MMS) and 3-methylcholanthrene |
Mouse Lymphoma Assay was conducted to evaluate the mutagenic potential of the KDD and to satisfy, in part, ISO 10993 Part 3: Tests for Genotoxicity, Carcinogenicity and Reproductive Toxicity At 14 days after cloning, neither saline nor DMSO KDD extracts caused a twofold increase in the background mutation frequency over the number of mutant colonies that arose spontaneously in the negative control. The KDD was nonmutagenic to mouse lymphoma cells |
Genotoxicity Mouse peripheral blood micronucleus study |
Test vehicles: 0.9% Sodium chloride injection, USP (SC) and sesame oil, NF (SO) Negative control: Vehicle without test material Positive control: MMS |
KDD extracts were evaluated for genotoxicity, to satisfy the requirements of, in part, ISO 10993 Part 3: Tests for Genotoxicity, Carcinogenicity and Reproductive Toxicity No statistically significant increase in cellular toxicity (% micronucleated reticulocytes) was observed following three consecutive days of dosing with saline and sesame oil extracts. The KDD was not genotoxic to the mouse, and there was no evidence of cellular toxicity |
Subchronic toxicity ISO muscle implantation study—2 weeks and 12 weeks |
Test implant: KDD (3 × 5 mm) cut in half Negative control article: HDPE cut into sections (5 × 10 mm) |
Muscle Implantation was conducted to determine the potential for irritation or toxicity of the KDD, satisfying the requirements of ISO 10993 Part 6: Tests for Local Effects after Implantation All test sites at 2 and 12 weeks, when viewed at low magnification showed no evidence of capsule formation or adverse reaction. Microscopic evaluation of tissue response indicated that the KDD was nonirritant The response to the KDD test article was not different from the negative control and is a nonirritant to rabbit muscle tissue |
Systemic toxicity Four and 26 weeks systemic toxicity study in rats following subcutaneous implant |
Test article: KDD Negative control article: HDPE cut into sections (3 × 10 mm) |
Surgical implantation of the KDD into the subcutaneous tissue of rats was performed to evaluate the potential sub chronic toxicity of the test article. Local irritation or toxicity at implant sites was also evaluated. Tests were based on requirements of ISO 10993 Part 11: Tests for Systemic Toxicity and the OECD Guideline for the Testing of Chemicals—Repeated Dose 28-day Oral Toxicity Study in Rodents, Document Number 407 No evidence of systemic toxicity from the subcutaneously implanted KDD as hematologic and clinical chemistry parameters were unaffected compared with the control. Macroscopic tissue reaction to the KDD was no different to the negative control. Microscopically, the test article was nonirritant |
3.2 Biomechanical testing results
3.2.1 Fatigue testing
- The KDD implant had not lost more than 10% of its original volume due to wear.
- The KDD implant had not split into more than three distinct pieces (not including wear debris).
- Each piece did not pass through a 5 mm diameter hole.
At 100 N preload, the mean displacement was 1.1 mm (SD 0.3) after 5 million cycles which decreased to 1.0 mm after 24 h without loading. At 600 N preload, the mean displacement was 2.8 mm (SD 0.3) after 5 million cycles which decreased to 2.6 mm after 24 h without loading (Table 3).
| Cycles (million) | Specimen 1 | Specimen 2 | Specimen 3 | Specimen 4 | Specimen 5 | Specimen 6 | Mean (mm) | Standard deviation |
|---|---|---|---|---|---|---|---|---|
| Displacement (mm) | Displacement (mm) | Displacement (mm) | Displacement (mm) | Displacement (mm) | Displacement (mm) | |||
| Fatigue testing | ||||||||
| 100 N 5 million | 0.0 | 0.0 | −0.1 | −0.1 | −0.2 | — | −0.1 | 0.1 |
| 600 N 5 million | −0.2 | 0.0 | −0.1 | −0.2 | −0.3 | — | −0.2 | 0.1 |
| Static creep testing | ||||||||
| After load | −0.18 | −0.20 | −0.26 | −0.15 | −0.27 | −0.21 | 0.05 | |
| Swell testing | ||||||||
| After load | −0.01 | 0.01 | 0.01 | −0.01 | −0.02 | −0.01 | 0.00 | 0.01 |
| Shock testing | ||||||||
| After third load | 0.6 | 0.5 | 0.5 | 0.3 | 0.4 | 0.4 | 0.4 | 0.1 |
| Aged fatigue testing | ||||||||
| 100 N 5 million | 0.53 | 0.49 | 0.44 | 0.45 | 0.55 | 0.46 | 0.49 | 0.05 |
| 600 N 5 million | 1.4 | 1.3 | 1.3 | 1.1 | 1.3 | 1.2 | 1.3 | 0.1 |
3.2.2 Wear testing
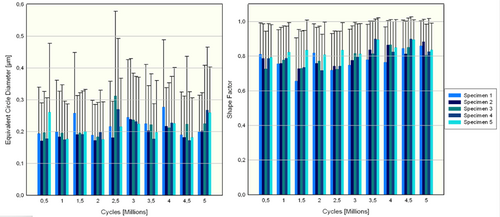
Two relatively large Barium-containing particles with equivalent circle diameters of 34.13 and 24.33 μm were found after 5 million cycles on Specimen 1. The remaining particles contained no traces of Barium (Figure A5).
EDX analysis of wear particles showed no trace of Barium, while Silicon, Gold, and Palladium were detected. The Gold and Palladium detection was due to contamination from the scanning electron microscopy analysis. The source of the Silicon particles could not be determined whether it came from the nucleus implant or the artificial annulus since both materials were similar in chemical composition.
3.2.3 Static creep testing
Loss of disc height is represented by a change in displacement, which was <0.2 mm or 1.5% of disc height across the period during which a constant load was applied (Table 3 and Figure A6).
3.2.4 Expulsion testing
Tests were configured for the worst-case scenario, using small implants which experienced the maximum moment when the annulus was in tension. No implant expulsions were observed for all KDD test articles after 100 000 cycles.
3.2.5 Swell testing
The KDD implanted in the surrogate annulus model did not exhibit significant swelling or shrinking when submerged in Ringer's solution for 48 h (Table 3 and Figure A6).
3.2.6 Shock testing
Mean permanent deformation of the KDD nucleus implant and annulus remained under 0.5 mm for each “shock” cycle and the implant did not fail during shock testing (Table 3 and Figure A6).
3.2.7 Aged fatigue testing
No failures of the implant were observed at test completion, and aging did not have a detrimental effect on the performance of the KDD nucleus implant.
3.3 Testing of silicone filler for estimation of shelf life
The testing addressed one aspect of long-term storage: will environmental factors such as ingress of airborne agents such as nitrous compounds diminish the activity of the platinum catalyst. This limited testing indicated there was no delayed or inhibited curing of the sterile silicone filler material stored in the FBA under ambient environmental conditions (Table 4 and Figure A5).
| Test article | T90 (min) | Scorch time (min) | Acceptance |
|---|---|---|---|
| 1 barrel + static mixer | 7.3 | 3.5 | Pass |
| 2 barrel + static mixer | 6.9 | 3.5 | Pass |
| 3 barrel + static mixer | 7.0 | 3.5 | Pass |
| 4 barrel + static mixer | 6.8 | 3.4 | Pass |
| 5 barrel + static mixer | 6.7 | 3.4 | Pass |
| 6 barrel + static mixer | 7.3 | 3.6 | Pass |
- Note: The test results from the six samples tested met specification supporting a shelf life of 6 months. The cure properties of the sterilized silicone filler material remained within specified limits (Scorch Time being a minimum of 1 min and T90 value below 10 min).
3.4 Thermodegradation testing of silicone filler
It was found that the silicone material intended for nucleus prosthesis can withstand dry heat temperatures of up to 400°C with a volatile material loss of <5% by weight.
3.5 Cadaver training
The mean times taken to establish a working cannula, removal of the herniated disc, size the disc cavity space using a radiopaque dye, deliver an implant jacket, and inject silicone polymer filler in the spinal disc space was improved from the first to the last cadaver training session (Table S2). Some minor deviations from the protocol during sizing the cavity and delivering the jacket at the first cadaver training session due to a lack of familiarity with the device which contributed to refining the human engineering aspect of the Kunovus system were recorded. Overall, the KDD system has been deemed effective in being able to carry out the implant procedure in a minimally invasive manner. With guidance from the surgical manual, surgeons were able to successfully deploy/implant the equipment in the level of L3–L4, L4–L5, and L5S1 during each cadaver training session. Preliminary results have shown all the devices have been successfully removed after implantation from the posterior procedure without any complications, including dura sac tear, the injury of the nerve root, and so forth.
3.6 Clinical study
A standard microdiscectomy was performed to remove the herniated disc in 45 min. After that, the KDD device was delivered through the pre-existing annulus tear in 20 min. Perioperative antibiotics are standard per St George Private Hospital surgical prophylaxis (Cefazolin 1 g iv for three doses). The patient was suggested to perform physiotherapy during postoperative rehabilitation and avoid activities that involve repetitive bending or twisting in the first 3 months. To assess the clinical utility of the KDD at this early stage, the results—although still preliminary—included here describe clinical experience and data of the first case as a part of the feasibility study. No intraoperative or postoperative vascular or neurological complications occurred in the first case (Figure 4). The preliminary clinical data showed decreases in both NRS and ODI scores after the KDD procedure. The preoperative NRS and ODI scores were 10 and 79.2, which decreased to 7 and 54 at 3 months postoperative, respectively. The KDD implantation procedure preserved the disc height of surgical level (DH from 8.2 mm preoperatively to 9.9 mm at 1 month postoperatively; and DHI from 0.20 preoperatively to 0.28 at 1 month postoperatively).
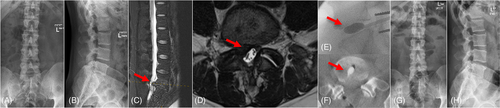
4 DISCUSSION
In this article, we evaluate a novel injectable in situ curing elastomeric device (KDD) using biocompatibility and biomechanical testing following the ISO standards. We found that all constituents of KDD are biocompatible without cell lysis and toxicity after biocompatibility testing. Subsequently, a series of mechanical tests were conducted to characterize the mechanical and wear behavior of the device, including no Barium-containing particles in fatigue test, no fracture of nucleus in static compression creep testing, no extrusion in expulsion testing, no swelling in swell testing, and no material failure in shock and aged fatigue testing.
Due to nonfixation to the endplate of nucleus augmentation device, the potential extrusion or expulsion in preformed elastomer18 and high contact stress to endplate of preformed mechanical device have become a significant concern for this type of device. Silicone is one of the promising candidates for an NP augmentation material. Following our previously published evidence from finite element analysis and in vitro mechanical testing,15, 16 we summarized the biocompatibility and mechanical properties to support the material to be successful as a nucleus augmentation device and be viable in a clinical setting19, 20 (Table 1).
4.1 Biocompatibility and cytotoxicity testing
The aim of biocompatibility testing is to determine the body's response to medical devices (e.g., direct or indirect), to ensure that the device is nontoxic and will not be rejected by the immune system. Toxicity may be caused by the material itself, its degradation products, or contaminants incorporated during the synthesis.
- Materials should be characterized to provide an understanding of the formulation, potential impurities, and extractable and to provide basis for specifications
- Leachable chemicals and degradation products should be considered in evaluating the toxicology of the device
- The availability of chemical extractables and degradation products to the patient when exposed to the device should be considered in designing testing programs.
- Changes in the composition of materials, manufacturing practices, or intended use of the device should be evaluated with respect to possible changes in toxicological effects on patients.
Akin to these requirements, the results of our study supported that the components of KDD are biocompatible and nontoxic using in vitro testing (e.g., contact, indirect contact, and/or extract cytotoxicity assays by different methods of interaction between the cells and the materials of the device) and in vivo testing (e.g., a generic small animal model for initial biological safety testing, a large animal model for assessing safety at the relevant anatomical site, etc).
In vitro testing reported that KDD is nontoxic using the definitions as follows: (1) a successful result will show cell growth uninhibited by the material and similar to a known noncytotoxic material22; and (2) the viable cell count should not reduce by more than 30% which is classified as being cytotoxic according to ISO 10993-5. This is consistent with the results of previous studies.23, 24 Most injectable nucleus augmentation devices were found to be noncytotoxic, as quantified by cell viability or proliferation, although different tests, cell types, and viability assays were used across the different research groups.23, 24
This study showed minimal inflammation, no production of fibrous scar tissue, and the presence of type 2 macrophages migrating into the KDD during in vivo testing. Some research groups have started in vivo small animal biocompatibility testing with in vivo subcutaneous implants of the injectable devices.14, 25-27 Two injectable devices as a subcutaneous implant showed the formation of fibrous tissue,25, 27 and one also resulted in an inflammatory response.26 The use of a crosslinker with one of the injectable devices was shown to cause the formation of a fibrous capsule.25 The same injectable device without the crosslinker did not result in a fibrous capsule formation. Some of these injectable devices exhibited positive cytotoxicity results and therefore emphasizes the importance of in vivo biocompatibility testing to understand the safety of the injectable devices.
4.2 Biomechanical testing
We developed a surrogate annulus model to add another dimension to spinal disc biomechanics studies. From the series of biomechanical (kinematic) tests, the KDD was able to restore the normal disc height. The functionality (biomechanics) and integrity (wear performance) of the device over its intended lifetime were evaluated during this study.
In vitro, mechanical testing should mimic in vivo conditions as closely as possible. This includes applying similar 3D kinematic ROMs (compression, flexion/extension, lateral bending, and axial rotation) which would be comparable to the ranges encountered in vivo, and loads that are similar in vivo. According to the standards used at the time of testing (e.g., ISO), a surrogate annulus model (developed per ASTM 2789-10 recommendations) was used to characterize various mechanical properties of the KDD. Early stages of the nucleus device development included the evaluation of a denucleated disc (the “degenerate” model) to demonstrate the effects of the absence of a nucleus initially, and subsequently to confirm the principle of nucleus replacement in degenerate discs. Static and dynamic creep testing was conducted using this in vitro model, and the results confirmed that the stability of a degenerate disc is compromised, with a large degree of deformation and loss in disc height. This is due to the abnormal stresses imparted to the disc annular walls in the absence of a nuclear core to distribute the load evenly.
However, the IVD functions such that the NP and AF work together to provide mechanical stability to the IVD joint. The annulus collagen fibers provide tensile strength resulting in spinal segment stability during physiological movement. The nucleus provides the internal hydrostatic pressure maintaining the disc height and supportive counter-pressure to the inner annulus wall.
A series of mechanical tests were conducted to characterize the mechanical and wear behavior of the device. Overall, the wear rate for each individual sample over the entire testing period was consistent, suggesting that little variation arose among the samples. All the surrogate annulus models remained intact without filler leakage during fatigue, static creep, expulsion, swell, and shock tests. Therefore, the results of these tests suggested that the KDD has sufficient strengths for the intended application.
4.3 Clinical outcomes of preformed elastomer
Previous studies on preformed elastomer showed that the average age of patients receiving a nucleus augmentation device was 35–45 years, and therefore the implant was expected to function for five to six decades.18, 28-33 The premise behind using a nucleus augmentation device is to restore mobility and salvage structures in a functionally suboptimal disc which would otherwise be sacrificed in more invasive spine surgeries. Although short-term (≤1 year) and mid-term (1–3 years) clinical results have been promising (pain scores, functional outcomes, disc height preservation, intraop, and postop complication rates), reoperation rates in the long-term remain a matter of concern, particularly for the mechanical and performed nucleus augmentation device.28-30 In 199 patients implanted with PDN and followed for a minimum of 4 years, endplate remodeling rate was 32%, subsidence rate was 26%, and reoperation rate was 27%.18, 31-33 For in situ curing implants, although conformity with the shape of the nucleotomized cavity has theoretical advantages in distributing loads to the adjoining structures, there is a dearth of long-term clinical follow-up data to show any translational benefits of this design principle.34, 35
The KDD may mimic native disc behavior in mechanical tests without cytotoxicity based on our biocompatibility testing, biomechanical testing, cadaver training, and feasibility study (Phase I), the need for further evaluation of the safety of the novel nucleus augmentation device through the clinical trial (Phase II and beyond) is warranted. Phase IIa (early safety study) study will be performed in a single center on 10–15 cases to assess how well the device works, and then Phase IIb (definite safety and early efficacy study) study will be performed in multicenter for the safety and early effectiveness of KDD, as we continue Phase I safety assessments with more biocompatibility and human cadaver studies.36, 37 It is quite likely that data from these studies may be adequate for regulatory submission and consequent approvals; However, once the efficacy is confirmed, Phase III of the clinical development of the device may be designed to assess the effectiveness and, thereby, its value in clinical practice through randomized controlled multicenter trials on larger patient groups in comparison with current gold standard treatment.
5 CONCLUSION
The biocompatibility testing confirmed that all constituents of KDD are biocompatible. The mechanical tests on the surrogate annulus model confirmed that the elastomeric nucleus device may mimic native disc behavior in mechanical tests, offering an effective way for treating LDH by way of Phase II and subsequent clinical trials or postmarket surveillance in the future.
AUTHOR CONTRIBUTIONS
The authors of this article all participated in the study design. All authors have read and approved this version of the article, and due care has been taken to ensure the integrity of the work. The material of this article is original research, and no part of this article has been previously published. The material has also not been submitted for publication elsewhere while under consideration. All authors have the appropriate permissions and rights to the reported data.
ACKNOWLEDGMENTS
The device(s)/drug(s) that is/are the subject of this article is/are being evaluated as part of a regulatory submission for the Kunovus Disc Device planned for the future.
FUNDING INFORMATION
Corporate/Industry funds from Kunovus Technologies were received to support this work. This project was funded by the BioMedTech Horizons program 2.0 (BMTH2.0) of the MTPConnect for Medical Research Future Fund (MRFF) of the Australian Government and St George Private Hospital. Alisha Sial is supported via an RTP scholarship of the Australian Government.
CONFLICT OF INTEREST STATEMENT
This work is an Australian Government supported small medium company start-up project, that is expected to work closely with academia for high-quality research with a risky commercial prospect, while building work-force capacity. Xiaolong Chen, Divya Bhargav, Johnathon Choi, Senori Perera, Cameron Dean, and Esther Apos are either employed or supported by Kunovus Technologies. Ashish D. Diwan conflicts as inventor are declared to IRB and he may receive possible royalties related to device for replacing nucleus and regenerating nucleus. Divya Bhargav is a shareholder. Saeed Kohan serves as a consultant in an honorary role till now and may receive consulting or research fees in the future. Richard Appleyard's institution receives MRFF support via Kunovus Technologies for Human Cadaveric Research.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article.
REFERENCES
APPENDIX