Development and Validation of a Predictive Risk Score for Blood Transfusion in Patients Undergoing Curative-Intent Surgery for Intrahepatic Cholangiocarcinoma
ABSTRACT
Background and Objectives
Among patients undergoing liver resection for intrahepatic cholangiocarcinoma (ICC), perioperative bleeding requiring blood transfusion is a common complication, yet preoperative identification of patients at risk for transfusion remains challenging. The objective of this study was to develop a preoperative risk score for blood transfusion requirement during surgery for ICC.
Methods
Patients undergoing curative-intent liver surgery for ICC (1990–2020) were identified from a multi-institutional database. A predictive model was developed and validated. An easy-to-use risk calculator was made available online.
Results
Among 1420 patients, 300 (21.1%) received an intraoperative transfusion. Independent predictors of transfusion included severe preoperative anemia (OR = 1.65, 95% CI 1.10–2.47), T2 category or higher (OR = 2.00, 95% CI 1.36–3.02), positive lymph nodes (OR = 1.75, 95% CI 1.32–2.32) and major resection (OR = 2.56, 95%CI 1.85–3.58). Receipt of blood transfusion significantly correlated with worse outcomes. The model showed good discriminative ability in both training (AUC = 0.68, 95% CI 0.66–0.72) and bootstrapping validation (C-index = 0.67, 95% CI 0.65–0.70) cohorts. An online risk calculator of blood transfusion requirement was developed (https://catalano-giovanni.shinyapps.io/TransfusionRisk).
Conclusions
Intraoperative blood transfusion was significantly associated with poor postoperative outcomes among patients undergoing surgery for ICC. The identification of patients at high risk of transfusion could improve perioperative patient care and blood resources allocation.
1 Introduction
Intrahepatic cholangiocarcinoma (ICC) represents approximately 15% of all primary liver tumors [1]. In addition, the incidence of ICC has been rising over the last several decades in the United States, as well as globally. Curative-intent liver resection is the primary curative-intent treatment option for patients with resectable, nonmetastatic ICC [2]. Despite advancements in surgical techniques, hepatic resection can still be associated with a high incidence of morbidity (22.5%) and mortality (2%–5%) [3]. In particular, peri-operative transfusion remains more common in the setting of a liver resection than many other complex gastrointestinal operations [4]. Although blood transfusions can be critical to maintain hemodynamic stability in the setting of acute hemorrhage, as well as treat postoperative anemia, perioperative blood transfusions have been associated with poor short- and long-term perioperative outcomes [5-8]. Blood transfusion has been associated with increased transmission of infections, transfusion-related acute lung injury, transfusion-associated circulatory overload, and disease recurrence [9-11]. Informing patients about the risk of a blood transfusion is a routine part of surgical informed consent [12, 13]. As part of the informed consent process, many patients frequently inquire as to the likelihood that a transfusion will be administered in the peri-operative period. The risk of transfusion with liver resection varies widely, however, ranging from 0% to 42% [14, 15].
To date, most predictive models of blood transfusion requirement during liver surgery have been developed based on single-center cohorts, thus limiting the generalizability to external patient populations [14]. In addition, many studies have focused on subspecialties such as cardiac and vascular surgical procedures [16, 17], or have examined risk related to transplantation or massive transfusion [18]. Surgical resection of ICC may be particularly challenging as many patients are often asymptomatic and present with large tumors necessitating major hepatectomy [19]. While several previous studies have proposed tools to predict transfusion risk before abdominal surgery [20, 21], no preoperative risk score has been proposed to date that specifically focuses on patients with ICC undergoing hepatic resection. Therefore, the objective of the current study was to utilize an international, multi-institutional cohort to develop a preoperative risk-score to stratify patients relative to the likelihood of receiving an intra-operative transfusion related to curative-intent liver surgery for ICC. An easy-to-use web-based calculator was developed and made available online to facilitate broad clinical applicability of the prediction model.
2 Materials and Methods
2.1 Study Population
Patients undergoing curative-intent liver resection for ICC between 1990 and 2020 were identified from a multi-institutional database of 15 tertiary institutions that comprise the International Intrahepatic Cholangiocarcinoma Study Group [22-25]. Patients undergoing palliative surgery were excluded. The Institutional Review Board of each participating institution approved this study.
2.2 Variables and Outcomes of Interest
Demographic, clinicopathologic, and treatment characteristics of interest included age, sex, American Society of Anesthesiologist (ASA) classification, body mass index (BMI), preoperative comorbidities such as history of cirrhosis or viral hepatitis (i.e., hepatitis B [HepB] or C [HepC]), preoperative laboratory markers, including carbohydrate antigen (CA) 19-9, total bilirubin, and hemoglobin (Hb) levels, preoperative anemia, tumor T- and N-stage, receipt of transfusion and extent of resection (i.e., minor or major).
Transfusion was defined as receipt of packed red blood cells (pRBCs) intraoperatively. The grade of preoperative anemia was categorized as “severe” if preoperative Hb levels were < 10 and < 11 g/dL for females and males, respectively; “mild” anemia was defined as Hb levels < 12 and < 13 g/dL for females and males, respectively [26-28]. The definition of T- and N-stage categories was based on the American Joint Committee on Cancer (AJCC) 8th edition staging manual [29]. The extent of liver resection was defined as major if three or more liver segments were resected, according to Couinaud's classification [30].
Postoperative outcomes of interest included hospital length-of-stay, complications within 90 days, postoperative mortality within 90 days, disease recurrence, and median overall survival (OS). OS was defined as the time interval between the date of hepatic resection and the date of death from any cause or last follow-up.
2.3 Statistical Analysis and Model Development
Continuous variables were reported as median (interquartile range [IQR]) and categorical variables were reported as frequencies (proportion, %). Continuous variables were compared using the Wilcoxon rank sum test. Categorical variables were compared using the chi-squared test or Fisher's exact test, as appropriate. Differences in survival among groups were assessed with the Kaplan–Meier method and compared with the log-rank test. The Multiple Imputation by Chained Equations (MICE) method was utilized to impute missing data [31].
Univariable and multivariable logistic regression analyses were used to assess the association of clinicopathologic variables with blood transfusion. Variables with a p-value < 0.1 in the univariable model were included in the multivariable regression analysis. Odds ratios (ORs) were presented with 95% confidence intervals (95% CI). The β-coefficients of the statistically significant variables (p < 0.05) in the multivariable model were used to develop a weighted risk score. The discrimination performance of the predictive model was evaluated using the area under the curve (AUC) of the receiver operating characteristics (ROC) curve. The model was internally validated using the bootstrapping resampling method with 5000 iterations. The c-index was calculated for the bootstrapping cohort, and the model's calibration was tested by plotting the predicted probabilities against the observed outcomes in the validation cohort. Patients with ICC undergoing liver surgery were categorized as low, medium, or high risk for intraoperative transfusion using the interquartile range values as cut-offs. All tests were two-sided, and a p-value < 0.05 was considered statistically significant. All statistical analyses were performed using R version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Baseline Characteristics of Patient Cohort
The final cohort consisted of 1420 patients undergoing liver resection for ICC who met inclusion criteria (Table 1). Median patient age was 62 years (IQR, 53.9–70.4) and median BMI was 25.3 kg/m2 (IQR, 22.4–28.2). Most patients were female (n = 648, 45.6%) and had an ASA class ≤ 2 (n = 852, 60.0%). A small subset of patients had cirrhosis (n = 152, 11.0%), HepB (n = 305, 21.0%), or HepC (n = 52%, 3.7%). Median CA 19-9 was 116.1 U/mL (IQR, 25.3–487.5), median total bilirubin was 0.7 mg/dL (IQR, 0.5–1.1), and median Hb was 12.8 g/dL (IQR, 11.9–14.0). Mild and severe preoperative anemia was present in 377 (27.0%) and 150 (11.0%) patients, respectively. Approximately one in two patients had T3 and T4 stage disease (n = 656, 46.6%), while 422 (30.0%) patients had nodal involvement (N1). A total of 862 (61.0%) patients underwent major hepatic resection.
| Variable | Overall N = 1420 |
Intraoperative transfusion | p-valuea | |
|---|---|---|---|---|
Not transfused N = 1120 |
Transfused N = 300 |
|||
| Age (years) | 62.0 (53.9, 70.4) | 61.0 (53.0, 70.0) | 62.0 (55.0, 71.0) | 0.11 |
| Female sex | 648 (46.0%) | 497 (44.0%) | 151 (50.0%) | 0.066 |
| BMI (kg/m2) | 25.3 (22.4, 28.2) | 25.3 (22.4, 28.1) | 25.3 (22.4, 28.6) | 0.89 |
| ASA-PS > 2 | 568 (40.0%) | 429 (38.0%) | 139 (46.0%) | 0.012 |
| Cirrhosis | 152 (11.0%) | 131 (12.0%) | 21 (7.0%) | 0.019 |
| Hepatitis B | 305 (21.0%) | 257 (23.0%) | 48 (16.0%) | 0.009 |
| Hepatitis C | 52 (3.7%) | 37 (3.3%) | 15 (5.0%) | 0.16 |
| CA 19-9 (U/mL) | 116.1 (25.3, 487.5) | 100.5 (21.7, 487.0) | 180.5 (39.8, 548.5) | 0.011 |
| Total bilirubin (mg/dL) | 0.70 (0.5, 1.1) | 0.7 (0.5, 1.0) | 0.8 (0.5, 1.1) | 0.016 |
| Preoperative hemoglobin (g/dL) | 12.8 (11.9, 14.0) | 12.9 (12.1, 14.1) | 12.4 (11.5, 13.7) | < 0.001 |
| Anemia class | 0.001 | |||
| Nonanemic | 893 (63.0%) | 726 (65.0%) | 167 (56.0%) | |
| Mild | 377 (27.0%) | 291 (26.0%) | 86 (29.0%) | |
| Severe | 150 (11.0%) | 103 (9.2%) | 47 (16.0%) | |
| AJCC T stage | < 0.001 | |||
| T1 | 307 (22.0%) | 273 (24.0%) | 34 (11.0%) | |
| T2 | 457 (32.0%) | 370 (33.0%) | 87 (29.0%) | |
| T3 | 647 (46.0%) | 474 (42.0%) | 173 (58.0%) | |
| T4 | 9 (0.6%) | 3 (0.3%) | 6 (2.0%) | |
| AJCC N1 stage | 422 (30.0%) | 297 (27.0%) | 125 (42.0%) | < 0.001 |
| Major resection | 862 (61.0%) | 624 (56.0%) | 238 (79.0%) | < 0.001 |
- Note: Continuous variables: median (IQR)/categorical variables: n (%).
- Abbreviations: AJCC, American Joint Committee on Cancer; ASA-PS, American Society of Anesthesiology Physical Status; BMI, body mass index; CA 19-9, carbohydrate antigen 19-9.
- a Bold values represent statistical significance.
3.2 Association of Clinicopathologic Characteristics With Blood Transfusion
Among patients undergoing curative-intent liver resection for ICC, 300 individuals received an intraoperative blood transfusion (21.1%), whereas 1120 (78.9%) patients were not transfused. Patients who received a blood transfusion had more frequently an ASA score > 2 (n = 139, 46.0% vs. n = 429, 38.8%; p = 0.012), higher preoperative CA 19-9 levels (180.5 [IQR, 39.8–548.5] vs. 100.5 [IQR, 21.7–487.0]; p = 0.011), AJCC T3 or T4 stage (n = 179, 60.0% vs. n = 477, 42.3%; p < 0.001) and metastatic lymph nodes (n = 125, 42.0% vs. n = 297, 27.0%; p < 0.001). Furthermore, patients who received intraoperative blood transfusion were less likely to have cirrhosis (n = 21, 7.0% vs. n = 131, 12.0%; p = 0.019) and HepB (n = 48, 16.0% vs. n = 257, 23.0%). In addition, patients who were transfused intraoperatively had lower preoperative Hb levels (12.4 [IQR, 11.5–13.7] vs. 12.9 [IQR, 12.1–14.1]; p < 0.001) and were more likely to have mild (n = 86, 29.0% vs. n = 291, 26.0%) or severe (n = 47, 16.0% vs. n = 103, 9.2%) anemia versus patients who were not transfused (p < 0.001) (Table 1). Major liver resection was more common among individuals who received an intraoperative blood transfusion (n = 238, 79.0% vs. n = 624, 56.0%; p < 0.001).
3.3 Association of Intraoperative Blood Transfusion With Postoperative Outcomes
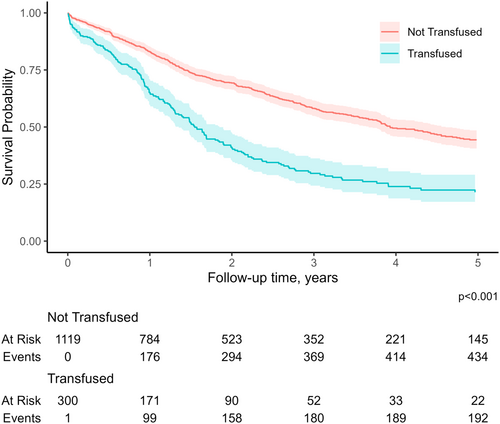
Patients who received intraoperative transfusion at the time of liver resection were more likely to have an extended length of stay (15 days [IQR, 10–27] vs. 11 days [IQR, 7–16]) and develop complications (n = 156, 52.0% vs. n = 313, 28.0%); patients who received an intraoperative transfusion also had a higher likelihood of postoperative mortality (n = 25, 8.3% vs. n = 15, 1.3%). Disease recurrence (n = 201, 67.0% vs. n = 662, 59.0%), as well as a worse median survival (15.2 months [IQR, 7.3–30.2] vs. 35.0 months [IQR, 14.2–59.6]) were more common among patients who received a blood transfusion compared with nontransfused patients (all p < 0.05, Table 2). In turn, receipt of intraoperative blood transfusion was associated with worse overall survival (p < 0.001, Figure 1).
| Variable | Overall N = 1420 |
Intraoperative transfusion | p-valuea | |
|---|---|---|---|---|
Not transfused N = 1120 |
Transfused N = 300 |
|||
| Length of stay (days) | 12.00 (7.00, 17.25) | 11.00 (7.00, 16.00) | 15.00 (10.00, 27.00) | < 0.001 |
| Complication | 469 (33%) | 313 (28%) | 156 (52%) | < 0.001 |
| Postoperative mortality | 40 (2.8%) | 15 (1.3%) | 25 (8.3%) | < 0.001 |
| Recurrence | 863 (61%) | 662 (59%) | 201 (67%) | 0.013 |
| OS (months) | 28.67 (11.84, 59.57) | 35.03 (14.23, 59.57) | 15.18 (7.33, 30.22) | < 0.001 |
- Note: Continuous variables: median (IQR)/categorical variables: n (%).
- Abbreviation: OS, overall survival.
- a Bold values represent statistical significance.
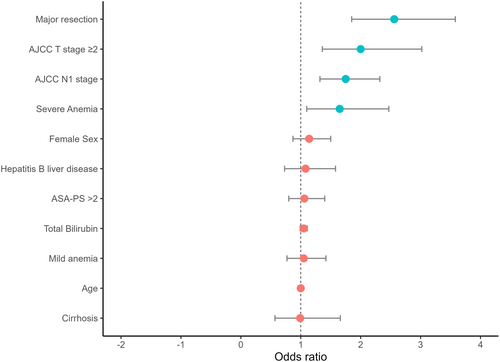
3.4 Independent Predictors of Intraoperative Transfusion and Risk Score Development
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-valuea | OR | 95% CI | p-valuea | |
| Age | 1.01 | 1.00, 1.02 | 0.091 | 1.00 | 0.99, 1.01 | 0.99 |
| Female sex | 1.27 | 0.98, 1.64 | 0.066 | 1.14 | 0.87, 1.50 | 0.33 |
| BMI | 1.00 | 0.98, 1.03 | 0.94 | |||
| ASA-PS > 2 | 1.39 | 1.07, 1.80 | 0.012 | 1.06 | 0.80, 1.40 | 0.68 |
| Cirrhosis | 0.57 | 0.34, 0.90 | 0.021 | 0.99 | 0.57, 1.66 | 0.98 |
| Hepatitis B | 0.64 | 0.45, 0.89 | 0.010 | 1.08 | 0.73, 1.58 | 0.69 |
| Hepatitis C | 1.54 | 0.81, 2.79 | 0.17 | |||
| CA 19-9 | 1.00 | 1.00, 1.00 | 0.47 | |||
| Total bilirubin | 1.08 | 1.02, 1.14 | 0.004 | 1.05 | 0.99, 1.11 | 0.076 |
| Anemia class | ||||||
| Nonanemic | ref | ref | ref | ref | ||
| Mild | 1.28 | 0.96, 1.72 | 0.094 | 1.05 | 0.77, 1.42 | 0.77 |
| Severe | 1.98 | 1.34, 2.90 | < 0.001 | 1.65 | 1.10, 2.47 | 0.014 |
| AJCC T stage ≥ 2 | 2.52 | 1.74, 3.75 | < 0.001 | 2.00 | 1.36, 3.02 | < 0.001 |
| AJCC N1 stage | 1.98 | 1.52, 2.58 | < 0.001 | 1.75 | 1.32, 2.32 | < 0.001 |
| Major resection | 3.05 | 2.27, 4.16 | < 0.001 | 2.56 | 1.85, 3.58 | < 0.001 |
- Abbreviations: ASA-PS, American Society of Anesthesiology Physical Status; BMI, body mass index; CA 19-9, carbohydrate antigen 19-9; CI, confidence interval; OR, odds ratio.
- a Bold values represent statistical significance.
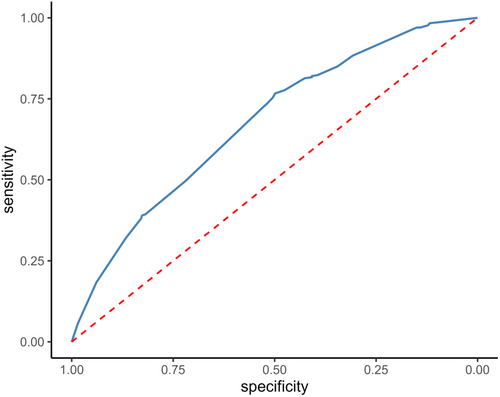
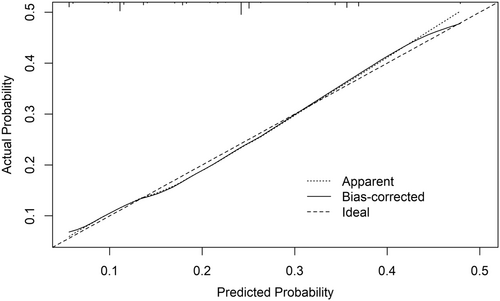
The risk score had an AUC of 0.68 (95% CI 0.66–0.72) in the training cohort (Figure 3) and its predictive accuracy was confirmed in the internal validation cohort with bootstrapping resamples (AUC 0.67 [95% CI 0.65–0.70]). The calibration plot indicated that the estimated probability and the observed frequency were in good agreement (Figure 4). Using the risk score, patients with ICC were categorized as being low, medium, or high risk of receiving intraoperative blood transfusion (Figure 5). The relationship between preoperative hemoglobin levels, T classification, and the probability of intraoperative blood transfusion was depicted with an interactive contour plot; in addition, an easy-to-use calculator was developed to predict the need for intraoperative blood transfusion (available at: https://catalano-giovanni.shinyapps.io/TransfusionRisk) (Figure 6).
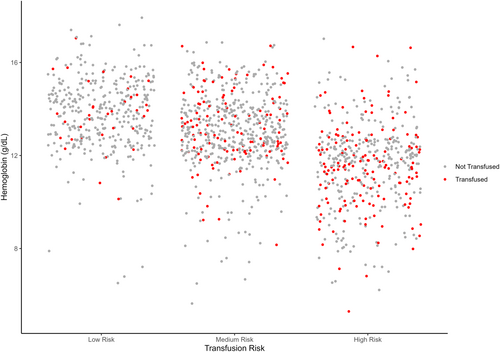
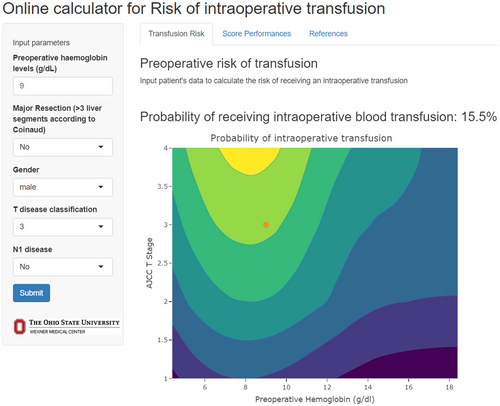
4 Discussion
ICC is a highly aggressive tumor with an increasing incidence worldwide [1]. Curative-intent hepatic resection is the main treatment for resectable, nonmetastatic ICC [2]. Despite substantial advancements in surgical techniques and perioperative strategies in recent years, prognosis even after hepatic resection remains poor with a median overall survival of 15–31 months [32]. Intraoperative bleeding is a common complication associated with liver surgery [3]. In turn, utilization of blood transfusion may be higher among patients with ICC who often present with large tumors in need of a major hepatic resection [15]. Notably, receipt of blood transfusion during hepatic surgery has been associated with transfusion-related complications, high mortality, and worse cancer-specific outcomes [33-36]. Meanwhile, there is an ongoing blood product shortage worldwide, highlighting the need for improved strategies of resource allocation and conservation [37]. The current study was important because we used an international, multi-institutional cohort to identify independent predictors of intraoperative blood transfusion among patients undergoing surgery for ICC. Of note, roughly one in five patients undergoing curative-intent liver resection for ICC received an intraoperative blood transfusion. The likelihood of a blood transfusion was associated with worse patient (i.e., anemia), tumor (i.e., T and N classification), as well as procedure (i.e., major resection) characteristics. In addition, receipt of an intraoperative transfusion was correlated with worse short- and long-term outcomes. A preoperative risk-score to estimate the probability of blood transfusion among patients undergoing curative-intent liver resection for ICC was developed and made available as a practical, online risk calculator.
Blood transfusion during surgery has been associated with adverse oncologic outcomes in various malignancies including gastric, pancreatic, hepatocellular, and lung cancer [36]. One proposed mechanism involves the immunomodulatory role of blood transfusion, which can decrease inteleukin-2 levels and cytotoxic T-cell activity, while elevating levels of immunosuppressive prostaglandins [36, 38-40]. Via these effects on the immune system, blood transfusion may mitigate tumor immune response and promote systemic inflammatory response; thus leading to increased morbidity and disease recurrence. Postlewait et al. reported that transfusion was associated with major complications and 90-day readmission [36]. In a different study, Kim et al. demonstrated that transfusion within 72 hours after surgery was an independent predictor of unplanned readmissions [41]. In the current study, patients who received intraoperative transfusion at the time of liver resection for ICC were more likely to have an extended length of stay (15 days [IQR, 10–27] vs. 11 days [IQR, 7–16]) and develop complications (n = 156, 52.0% vs. n = 313, 28.0%); patients who received an intraoperative transfusion also had a higher likelihood of postoperative mortality (n = 25, 8.3% vs. n = 15, 1.3%). Other data have suggested that peri-operative blood transfusions can lead to worse oncologic outcomes [5]. In fact, peri-operative blood transfusion has been associated with a 5-year OS almost 2.5 times lower than in patients who did not receive a transfusion [6-8]. In the current study, receipt of an intra-operative transfusion at the time of liver resection for ICC was associated with increased disease recurrence, as well as worse survival.
To date, few predictive models have been developed to assess the potential need for blood transfusion among patients undergoing hepatic resection [42-44]. Additionally, most existing models were developed using cohorts from a single institution, thus limiting their validity among more diverse patient populations [14]. For example, Lemke et al. evaluated three, single-center transfusion risk scores, in addition to developing and validating a simplified three-point risk score [44]. The three-point risk score incorporated preoperative anemia, primary liver cancer diagnosis, and major resection as predictive factors. This score had an AUC of 0.66 (95% CI 0.63–0.69), which was comparable to the reported accuracy of other published prediction tools (Cockbain et al. [45]: 0.66, 95% CI 0.63–0.69; Sima et al. [46]: 0.66, 95% CI 0.63–0.70; Pulitanò et al. [43]: 0.68, 95% CI 0.64–0.71). In a different, single-institution, study of patients undergoing hepatic resection for HepB-related HCC, Wang et al. developed a risk score for transfusion based on extent of resection, extrahepatic procedures, hemoglobin and platelet levels (AUC 0.74) [42]. In the current study, independent predictors of intraoperative blood transfusion related to ICC included extent of preoperative anemia, AJCC T and N disease, as well as extent of resection. Utilizing these factors, a risk score to predict the likelihood of an intraoperative transfusion was developed. Of note, the score performed comparably to other reported prediction tools in both the training (AUC 0.68) and validation (AUC 0.67) cohorts with good calibration [43, 45, 46]. The risk score was able to stratify patients into three groups; low, medium, and high risk for intraoperative blood transfusion requirement.
The availability of a tool to predict the probability of blood transfusion in the preoperative setting has important clinical implications. A tool to guide the likelihood of transfusion may help inform which patients may benefit from referral to the blood management program [47]. Preoperative blood ordering involves screening for red cell antibodies and/or crossmatch with specific units of packed red blood cells in anticipation of a possible transfusion [48]. Excessive crossmatch of blood before the operation can overburden blood bank personnel, as well as lead to outdating of blood, depletion of blood bank resources, and increased hospital costs [49]. Our group has previous reported that more than one in four patients receive crossmatch orders that exceeded national guidelines. In addition, there is a marked provider variation among surgeons associated with ordering of blood for crossmatch in the preoperative setting [48]. More accurate prediction of transfusion needs in the preoperative setting may reduce excess crossmatch ordering before surgery. In addition, a better understanding of the likelihood of an intraoperative transfusion may inform the need of autologous blood donation, as well as making cell-saver available for autotransfusion in the operating room [50, 51]. Furthermore, the identification of patients at higher risk for blood transfusion could aid in better allocating blood product resources, avoid blood product waste, and, thus, improve cost-effectiveness. The availability of the tool in an easy-to-use web calculator can facilitate its clinical application (https://catalano-giovanni.shinyapps.io/TransfusionRisk).
The present study should be interpreted in light of several limitations. Due to the retrospective design of the study, selection and reporting biases were possible. Although the current analysis considered many factors related to transfusion risk, other factors such as surgical technique, the use of blood flow management strategies, and the experience of the Anesthesia team may have influenced the results. In fact, even though the multi-institutional nature of the cohort was a strength, perioperative clinical and surgical management, as well as intraoperative transfusion practices may have varied among different institutions. Further validation of the predictive risk score in external cohorts is therefore necessary.
5 Conclusions
In conclusion, one in five patients undergoing resection of ICC received an intraoperative blood transfusion. Blood transfusion was associated with worse clinicopathologic characteristics and poor postoperative outcomes. A preoperative risk score to predict the likelihood of a blood transfusion during surgery for ICC was developed based on patient, tumor, and procedure-related factors. The tool had good accuracy and was made available via an on-line calculator to assist clinicians. The preoperative risk stratification of patients relative to likelihood of a transfusion can assist informed consent, as well as help with peri-operative planning relative to curative-intent liver resection for ICC.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Synopsis
Patients undergoing liver resection surgery for intrahepatic cholangiocarcinoma have a high risk of perioperative bleeding. Intraoperative blood transfusions are associated with worse postoperative outcomes. Our study aimed to develop a preoperative risk calculator to identify patients at higher risk for intraoperative transfusion, aiding in better blood products allocation.




