Tranexamic acid use in sarcoma surgery patients: A systematic review and meta-analysis
Abstract
Introduction
Perioperative bleeding increases morbidity and mortality in sarcoma patients. Tranexamic acid (TXA), an antifibrinolytic, is widely utilized in non-sarcoma orthopaedic surgeries, but its adoption in sarcoma surgery is hindered by concerns about thrombotic events.
Methods
Searches in Ovid MEDLINE, EMBASE, and CENTRAL were performed without date restrictions. Inclusion criteria encompassed sarcoma patients undergoing surgery with TXA intervention. Two authors independently screened studies, resolved conflicts, and assessed biases.
Results
Eight studies met inclusion criteria, comprising 2142 patients. TXA administration varied in dose and timing across studies. Meta-analysis revealed significantly reduced mean blood loss with TXA of −462.5 mL ([95% confidence interval [CI: −596.7, −328.31], p < 0.001) but no difference in transfusion rates (odds ratio [OR] = 0.51 [95% CI: 0.14–1.89]) or venous thromboembolism events (OR = 0.93 [95% CI: 0.40, 2.16]). Study biases were predominantly moderate to high due to retrospective designs and lack of control for confounders. Quality of reporting varied, with limitations identified in outcome reporting and effect size estimation.
Conclusions
Despite evidence of reduced blood loss, the absence of prospective studies limits conclusive recommendations on TXA use in sarcoma surgery. Further research is warranted to determine optimal TXA regimens and assess safety concerns regarding thrombotic events in this patient population.
1 INTRODUCTION
Surgical resection is the mainstay of the curative management of bone and soft tissue sarcomas. Given the complexity of the intervention, surgery often requires extensive soft tissue dissection with high rates of blood loss. The rates of intraoperative and postoperative blood transfusions has been reported between 20% and 40% depending on case complexity and tumor location.1-4 Perioperative bleeding has been associated with higher morbidity and mortality within the sarcoma population.5
Tranexamic acid (TXA) is an antifibrinolytic drug that has been widely studied and utilized in non-sarcoma orthopaedic surgery, cardiac surgery and trauma surgery.6 A meta-analysis of TXA use in orthopaedic trauma surgery demonstrated significantly less blood loss and risk of blood transfusion in patients that were administered TXA, compared to controls, with no significant differences in symptomatic thromboembolic events.6 Similarly, a recently published trial of patients undergoing major noncardiac surgery demonstrated that bleeding events were significantly lower with TXA compared to placebo. However, there has been slower uptake in the utilization of TXA in sarcoma patients given the high risk of perioperative thrombotic events.7 Patients with active malignancy are at a sevenfold risk of venous thromboembolism owing to the hypercoagulable state associated with malignancy and systemic cytotoxic therapies.8 Furthermore, sarcoma patients are subject to long operative times, intraoperative vessel manipulation, and periods of prolonged postoperative immobilization, all factors associated with increased rates of thrombotic events.9-11 While TXA has demonstrated safety and efficacy in other orthopaedic procedures, orthopaedic sarcoma surgery represents a unique subset of patients, and its role has not been well-established.
We conducted a systematic review and meta-analysis to examine the efficacy and safety of intraoperative TXA administration in patients undergoing sarcoma surgery. Specifically, our aim was to answer the following questions: (1) Does TXA administration reduce blood loss and transfusion rates in sarcoma patients undergoing surgery? (2) Does TXA administration increase the rates of postoperative thrombotic events in sarcoma patients undergoing surgery? (3) What is the quality of evidence evaluating the use of TXA in sarcoma patients?
2 METHODS
2.1 Protocol and registration
This systematic review and meta-analysis followed the preferred reporting for systematic reviews and meta-analyzes (PRSIMA) and Cochrane Collaboration guidelines for performing and reporting systematic reviews.12 This review was prospectively registered in the PROSPERO registry, Id: CRD42024519943.
2.2 Search strategy and study selection
A systematic review of Ovid MEDLINE, EMBASE, and CENTRAL was performed for relevant studies on December 12, 2023. In conjunction with the research librarian, the search strategy was developed uniquely for each medical database by combining exploded medical indexing terms (MeSH® terms for MEDLINE and Central, EMTree® terms for EMBASE) and keywords using Boolean operators “OR” and “AND.” A complete list of search terms used for each database can be found in Appendix I. There were no date restrictions imposed upon the search, but the search was limited to articles published in the English language.
The inclusion and exclusion criteria were defined a priori. Randomized control studies, cohort studies and case-control studies were eligible for inclusion. The inclusion criteria were: (1) sarcoma patients undergoing surgical intervention, (2) TXA was utilized as an intervention perioperatively, (3) study was published in the English language. Studies were excluded if they focused on retroperitoneal sarcomas or metastatic bone disease exclusively. Screening questions and forms for title, abstract and full-text screening were developed based on the inclusion and exclusion criteria. Two authors (VG and HF) independently screened the titles and abstracts identified in the literature searches in duplicate. The full text of all articles identified as potentially eligible were be reviewed in duplicate by authors. Disagreements were reconciled through a consensus meeting with the Principal Investigator (AG). A PRISMA flow diagram was utilized to demonstrate the number of included and excluded studies.
2.3 Data extraction and statistical analysis
Data extraction was performed utilizing an online collaborative data-extraction form that was created a priori and tested before data extraction. The following data was extracted: study design, demographic data, intervention and comparator details and length of follow-up. The rates of perioperative transfusions, estimated blood loss and venous thromboembolic (VTE) were recorded.
Data was assessed for appropriate quantitative pooling. Pooled effect estimates were calculated using SPSS. Heterogeneity was assessed using the Chi-square test and I2 statistic. An I2 value of 0%–40% was considered low heterogeneity, 40%–60% as moderate and greater than 60% as high.13 The pooled effect estimates are presented as odds ratios with associated 95% confidence intervals (CI) for dichotomous outcomes. Mean differences (MD) with 95% CIs were utilized for continuous data. As per the Cochrane Handbook, a predefined algorithm was used to estimate standard deviation (SD) if the study did not report SD.
2.4 Study appraisal
Author pairs independently graded the methodological quality of each included study using the risk of bias and quality of data form developed (see Appendix I for details). The form was an adaptation of the Cochrane Collaboration's risk of bias tool, risk of bias in non-randomized studies of interventions tool and the STROBE Checklist.14, 15 If there was insufficient information in the full text article to judge a question, the response was marked as “unclear.” Any disagreements between the author pairs were reconciled through a consensus meeting with the Principal Investigator (AG).
3 RESULTS
3.1 Study characteristics
Our search of databases yielded 974 potentially eligible studies, with no additional records identified through reference screening. Following duplicate removal, 924 titles remained for screening. A total of 907 studies were excluded following title and abstract screening, leaving 17 articles remaining for full-text screening. Nine articles were excluded at this stage due to patients not having sarcoma, patients not being given TXA, one article not reporting on an outcome of interest, one article not in the English language, and one article in which the full-text was not able to be obtained, even after contacting the authors. Study selection is presented in Figure 1.
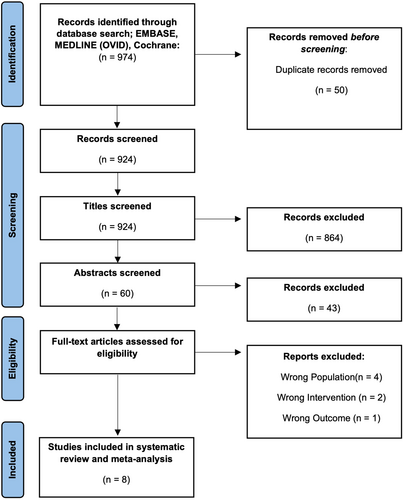
Eight studies met the inclusion criteria for this systematic review and meta-analysis, comprising a total of 2142 patients (Table 1).16-23 No study was published before 2020, and the majority of studies (n = 7) were retrospective cohort design of a single center. One study was a secondary analysis of a multi-center randomized controlled trial.
| Study | Year | Study design | N | Mean age (SD)a | Type of sarcoma | Location of sarcoma | Thromboprophylaxis | Dose and timing of TXA | Blood loss calculation | Length of follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Atalay et al.16 | 2020 | Retrospective cohort | 46 | 60.7 (14.7) | Bone and oligometastatic bone disease | Lower limb | 4000IU LMWH once daily | 15 mg/kg IV pre-operatively | Blood volume, whole blood and sponge count | NR |
| Foster et al.17 | 2023 | Retrospective cohort | 1099 | 55.7 (20.2) | Bone and soft tissue | Pelvis and upper and lower limb | None, Antiplatelet, DOAC, P2Y12 inhibitor, LMWH | 1 G at incision, 1 G before wound closure | NR | 90 days |
| Hasse et al. | 2020 | Retrospective cohort | 90 | 58.0 (17.2) [TXA Group] 54.7 (18.2) [Non-TXA Group] | Bone and oligometastatic bone disease | Lower limb | DOAC, LMWH, Mechanical for 28 days | 1 G topical before wound closure | Estimated from pre and post operate Hb and transfusions given | 6 weeks |
| Hess et al. | 2023 | Retrospective cohort | 98 | 57 (37) | Bone and soft tissue | Upper and lower limb | LMWH (weight based), LMWH (40 mg OD), aspirin (81 mg BID) or SC heparin (5000 units TID) outpatient | 1 G IV at incision and additional 1 G IV before closure at surgeons’ disclosure | Estimated, unclear | 6 Weeks |
| Oyama et al.20,b | 2022 | Retrospective cohort - propensity score matching | 88 | 45.1 (22.1) [TXA Group] 47.6 (20.7) [Non-TXA Group] | Bone and soft tissue | Upper limb, lower limb, trunk | None | 1 G IV preoperatively and 500 mg postoperatively (+500 mg if op time >3 h) | Hb balance method33 | 90 days |
| Sabharwal et al.21 | 2023 | Secondary analysis of RCT | 604 | 41.2 (21.9) | Bone, soft tissue and oligometastatic bone disease | Lower limb | LMWH, DOAC, Heparin, Coumadin, None | NR (IV or topical) | NR | 1 year |
| Sofulu et al.22 | 2021 | Retrospective cohort | 56 | 15.2 (3.0) [TXA Group] 14.3 (2.6) [Non-TXA Group] | Bone | Lower limb | 4000IU LMWH once daily (21 days) | 15 mg/kg IV Preoperatively | Hb balance method33 | 6 weeks |
| Tsantes et al.23 | 2021 | Retrospective cohort | 61 | NR | Bone and oligometastatic bone disease | Lower limb | LMWH | 1.5 G IV preoperatively and 1.5 G postoperatively locally | Hb balance method33 | 3 months |
- Abbreviations: BID, twice daily; DOAC, direct oral anticoagulant, G, gram; Hb, haemaglobin; IV, intravenous; LMWH, low molecular weight heparin; NR, not reported; OD, once daily; RCT, randomized controlled trial; SD, standard deviation; TID; three times daily; TXA, tranexamic acid.
- a If the study did not report mean age for the entire cohort, the separate group data are reported.
- b Only wide excision data was extracted as the types of surgeries were analyzed separately.
3.2 Patient demographics
The mean age of included patients was 45 years old (SD ± 17) (n = 7 studies). Follow-up ranged from 6 weeks to 1 year postoperatively. All studies included patients with bone sarcomas, with four studies also including patients with soft-tissue sarcomas and four studies also including patients with oligometastatic bone disease. All studies included sarcomas of the lower limb, with three studies also including patients with upper extremity sarcomas (Table 1).
Thromboprophylaxis varied among the included studies and between patients in included studies from a range of no thromboprophylaxis to mechanical thromboprophylaxis, direct oral anticoagulants, aspirin and low molecular weight heparin.
3.3 TXA use
The dose and timing of TXA also varied among studies. Most commonly 1 G IV was given preoperatively (n = 3), however, dosing regimens of 1.5 G IV and 15 mg/kg IV were also reported. Four studies also reported additional TXA administration at wound closure, with two studies including this additional dose based on surgeons' disclosure. One study reported the use of a single 1 G topical dose of TXA before wound closure.
3.4 VTE rates
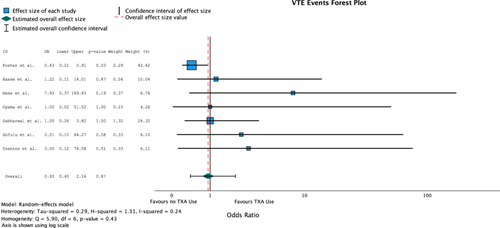
Seven of the eight included studies evaluated postoperative VTE events (n = 2096). One study reported a significant increase in VTE events with TXA compared to no TXA groups (odds ratio [OR] = 2.22 [95% CI: 1.03, 4.79], p-value: 0.042). Overall, 18 VTE events were reported in the TXA groups (n = 679) for a rate of 2.7% and 29 VTE events were reported in the non-TXA groups for a rate of 2.0%. Heterogeneity was low for all combined studies (I2 = 24%, Q = 5.90, p = 0.43). The combined odds ratio did not reveal any significant difference in TXA use on VTE events (OR = 0.93 [95% CI: 0.40, 2.16], p = 0.87) (Table 2) (Figure 2).
| Study | Year | N | TXA | No TXA | ||
|---|---|---|---|---|---|---|
| No VTE events | VTE events | No VTE events | VTE events | |||
| Foster et al.17 | 2023 | 1099 | 308 | 14 | 762 | 15 |
| Hasse et al. | 2020 | 90 | 33 | 1 | 54 | 2 |
| Hess et al. | 2023 | 98 | 59 | 0 | 37 | 2 |
| Oyama et al.20 | 2022 | 88 | 44 | 0 | 44 | 0 |
| Sabharwal et al.21 | 2023 | 604 | 162 | 3 | 431 | 8 |
| Sofulu et al.22 | 2021 | 56 | 25 | 0 | 30 | 1 |
| Tsantes et al.23 | 2021 | 61 | 30 | 0 | 30 | 1 |
- Abbreviations: TXA, tranexamic acid; VTE, venous thromboembolic (including deep vein thrombosis and pulmonary embolism).
3.5 Transfusion events
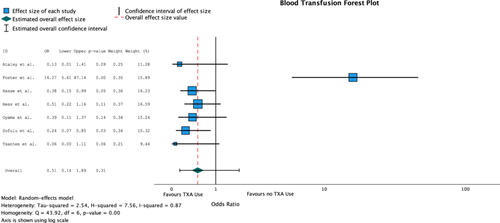
Seven of the eight included studies evaluated blood transfusion requirements (n = 1538). Four studies reported a significant difference in transfusion requirements between the TXA and non-TXA groups. Overall, 109 patients required a blood transfusion in the TXA groups (n = 529) for a rate of 3.4% and 146 patients required a blood transfusion in the non-TXA groups for a rate of 16.9%. Heterogeneity was high for all combined studies and was statistically significant (I2 = 87%, Q = 43.92, p = 0.00). The combined odds ratio did not reveal any significant difference in TXA use on rates of blood transfusions (OR = 0.51 [95% CI: 0.14, 1.89], p = 0.31) (Table 3) (Figure 3).
| Study | Year | N | TXA | No TXA | ||
|---|---|---|---|---|---|---|
| # Patients not requiring blood transfusions | # Patients requiring blood transfusions | # Patients not requiring blood transfusions | # Patients requiring blood transfusions | |||
| Atalay et al.16 | 2020 | 46 | 3 | 12 | 1 | 30 |
| Foster et al.17 | 2023 | 1099 | 297 | 25 | 773 | 4 |
| Hasse et al. | 2020 | 90 | 26 | 8 | 31 | 25 |
| Hess et al. | 2022 | 98 | 37 | 22 | 18 | 21 |
| Oyama et al.20 | 2023 | 88 | 40 | 4 | 35 | 9 |
| Sofulu et al.22 | 2021 | 56 | 11 | 14 | 5 | 26 |
| Tsantes et al.23 | 2021 | 61 | 6 | 24 | 0 | 31 |
- Abbreviation: TXA, tranexamic acid.
3.6 Estimated blood loss
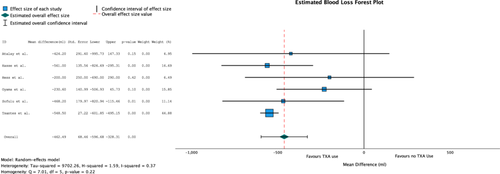
Six of the eight included studies evaluated estimated blood loss (n = 439). Five studies reported a significant difference in blood loss between the TXA and non-TXA groups. Overall, average blood loss in the TXA group was 1020 mL (SD ± 535.6) and 1425.4 mL (SD ± 655.1) in the non-TXA group. Heterogeneity was low for all combined studies (I2 = 37%, Q = 7.01, p = 0.22). The combined analysis of the MD of these studies revealed a significant difference in TXA use on mean blood loss of −462.5 mL ([95% CI: −596.7 to −328.31], p < 0.001) (Table 4) (Figure 4).
| Study | Year | N | TXA | No TXA | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Atalay et al.16 | 2020 | 46 | 1746.7 | 1233.3 | 2170.9 | 742.2 |
| Hasse et al. | 2020 | 90 | 981 | 290 | 1542 | 756 |
| Hess et al. | 2022 | 98 | 500 | 650 | 700 | 1750 |
| Oyama et al.20 | 2023 | 88 | 320 | 534 | 550.6 | 767.75 |
| Sofulu et al.22 | 2021 | 56 | 1247.5 | 300.9 | 1715.7 | 857 |
| Tsantes et al.23 | 2021 | 61 | 1324.5 | 101.75 | 1873 | 110.5 |
- Abbreviations: SD, standard deviation; TXA, tranexamic acid.
3.7 Risk of bias
Out of the eight studies reviewed, seven were retrospective cohort studies conducted at a single center, while one study involved a secondary analysis of a multicenter randomized controlled trial. Most studies effectively outlined participant characteristics before selection, resulting in a low risk of selection bias. However, given the nature of these studies, confounding bias was rated as moderate or higher in each case, particularly when appropriate methods to control for confounders were not utilized. Two studies that reported additional TXA administration based on surgeons' disclosure and one study failed to adequately describe the dosing of TXA, leading to a moderate risk of intervention bias. Performance bias was consistently low across all studies due to the nature of the outcomes examined. However, detection bias could not be evaluated for any of the studies. Attrition bias varied among included studies, as some excluded participants due to missing data. All studies demonstrated a low risk of reporting bias, as each underwent a single analysis. Sample size and method of analysis biases were not applicable to the majority of studies, with only one study being rated as low risk in this regard. Moreover, no conflicts of interest were identified within any of the included studies. The comprehensive assessment of biases is summarized in Table 5.
| Study | Atalay et al.16 | Foster et al.17 | Hasse et al. | Hess et al. | Oyama et al.20 | Sabharwal et al.21 | Sofulu et al.22 | Tsantes et al.23 |
|---|---|---|---|---|---|---|---|---|
| Selection bias | Low risk | Low risk | Low risk | Moderate risk | Low risk | Low risk | Low risk | Low risk |
| Confounding bias | Serious risk | Moderate risk | Moderate risk | Moderate risk | Critical Risk | Moderate risk | Critical risk | Moderate risk |
| Intervention bias | Low risk | Low risk | Low risk | Moderate risk | Moderate risk | Moderate risk | Low risk | Low risk |
| Performance bias | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Detection bias | No information | No information | No information | No information | No information | No information | No information | No information |
| Attrition bias | Moderate risk | Low risk | Moderate risk | Serious risk | Low risk | Low risk | Serious risk | Moderate risk |
| Reporting bias | Low bias | Low bias | Low bias | Low bias | Low bias | Low bias | Low bias | Low bias |
| Sample size | NA | NA | NA | NA | NA | Low risk | NA | NA |
| Method of analysis | NA | NA | NA | NA | NA | Low risk | NA | NA |
| Conflicts of interest | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
- Abbreviation: NA, not applicable.
3.8 Quality of reporting
All studies provided adequate background information and rationale for the study and detailed interventions and eligibility criteria. Furthermore, all the studies adequately defined outcomes, including describing the collection of blood or the hospital protocol for blood transfusions. Quality of data declined in assessment of the results, as sources of bias were not adequately addressed, and most studies failed to report absolute and relative effect sizes. However, limitations and generalizability of every study was clearly discussed. Quality of reporting for included studies in presented in Table 6.
| Atalay et al.16 | Foster et al.17 | Hasse et al. | Hess et al. | Oyama et al.20,* | Sabharwal et al.21 | Sofulu et al.22 | Tsantes et al.23 | |
|---|---|---|---|---|---|---|---|---|
| B25 | Y | Y | Y | Y | Y | Y | Y | Y |
| B26 | Y | Y | Y | Y | Y | Y | Y | Y |
| B27 | Y | Y | Y | Y | Y | Y | Y | Y |
| B28 | Y | Y | Y | Y | Y | Y | Y | Y |
| B29 | NA | NA | NA | Y | Y | NA | NA | NA |
| B30 | Y | Y | Y | Y | Y | Y | Y | Y |
| B31 | Y | Y | Y | Y | Y | Y | Y | Y |
| B32 | N | Y | Y | Y | Y | Y | N | N |
| B33 | Y | N | N | N | Y | Y | Y | Y |
| B34 | N | Y | Y | Y | Y | Y | Y | N |
| B35 | Y | Y | Y | Y | Y | Y | Y | Y |
| B36 | Y | Y | Y | Y | Y | Y | Y | Y |
| B37 | N | Y | N | N | N | N | N | N |
| B38 | Y | Y | Y | Y | Y | Y | Y | Y |
| B39 | Y | Y | Y | Y | Y | Y | Y | Y |
| B40 | Y | Y | Y | Y | Y | Y | Y | Y |
| B41 | Y | Y | Y | Y | Y | Y | Y | Y |
- Note: Y, yes; N, no; NA, not applicable. Questions follow Appendix I quality of data collection form based on the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) checklist.
4 DISCUSSION
The results of the current review demonstrate that intraoperative TXA administration significantly reduces blood loss in orthopaedic sarcoma surgery. There was no difference in the rates of VTE or perioperative transfusions based on the available literature. The overall quality of the existing literature is low, with no prospective studies available to date.
Large trials in both trauma and obstetrics have demonstrated that TXA lowers the risk of mortality compared to placebo.24, 25 In patients undergoing major noncardiac surgery, Devereaux et al.26 demonstrated that intravenous TXA administration lowered the relative risk of major bleeding events by 25%. The rates of blood transfusions in sarcoma surgery are substantially higher than other orthopaedic surgery in part due to larger surgical interventions, tumoral angiogenesis and preoperative malignancy associated anemia.27 In the current study, estimated blood loss was significantly decreased in patients receiving TXA but no overall differences in transfusion rates were observed. The lack of difference in transfusion rates was likely attributable, in part, to the largest included study by Foster et al.17 They reported higher transfusion rates in the TXA group, likely secondary to selection bias given that patients with larger, pelvic based tumors had higher rates of TXA utilization. The largest included study by Foster et al.17 did not report on estimated blood loss in their study, which may have contributed to the pooled analysis that demonstrated overall reduction in blood loos with TXA administration.
A recent meta-analysis of 8077 patients with extremity bone or soft-tissue sarcoma reported overall VTE rates of 2.9%.6 This rate is slightly higher than the VTE rates we observed in our study, of 2.7% and 2.0% for TXA and non-TXA groups, respectively. This may suggest underreporting in our included studies, as VTE were not always the primary outcome of interest and previous studies have described up to 12% of VTEs to be asymptomatic.
While the safety of TXA has been demonstrated across several populations, there remains concern among high-risk patient populations.17, 26 Although there is some data from the arthroplasty and metastatic bone disease literature suggest that TXA reduces VTE, this was not borne out in the current study.6, 26, 28 Devereaux at al.26 randomized 9535 patients undergoing noncardiac surgery who were at risk for postoperative bleeding and cardiovascular complications. They were not able to establish noninferiority of TXA compared to placebo with respect to cardiovascular complications.26 Similarly, a meta-analysis of TXA use in orthopaedic trauma surgery demonstrated no significant difference in risk of symptomatic thromboembolic events (OR: 0.968; 95% CI: 0.530–1.766) with TXA use.6 The results of the current meta-analysis demonstrate no difference in VTE events between the two groups. In the largest study included in this meta-analysis, Foster et al.17 found that TXA administration was an independent predictor of postoperative pulmonary embolism.17 Given the small sample size and retrospective nature of the included study, further prospective data is required to ensure safety in the sarcoma population.
Across other areas of orthopaedic surgery, there has been a high adoption of intraoperative TXA use as an effective and safe hemostatic agent.29 The use of TXA in lower extremity joint replacement surgery has been adopted into clinical practice guidelines and widely endorsed by various organizations.30 Despite the high rates of blood loss and transfusion rates in sarcoma surgery, the utilization of TXA has been relatively low. A secondary analysis of the PARITY randomized controlled trial in sarcoma patients undergoing resection and endoprosthetic reconstruction demonstrated that only 27% patients received intraoperative TXA.21 This is likely in part due to the ongoing concern for VTE in this high-risk population and the lack of high-quality evidence to guide practice.
4.1 Limitations
The variability between studies in respect to dosing and timing of TXA administration, thromboembolic use, and patient populations limits the interpretation of the pooled analyzes, particularly for blood transfusion events in which the heterogeneity was high. Furthermore, transfusion events were evaluated on a binary basis, significantly limiting the interpretation of the benefits of TXA on transfusion rates. Moreover, the lack of prospective studies provides a low quality of evidence, thereby, limiting the interpretation and development of recommendations on TXA use in extremity sarcoma surgery.
5 CONCLUSIONS
In this systematic review we found a lack of prospective evidence on the efficacy and safety of intraoperative TXA administration in patients undergoing sarcoma surgery. Furthermore, no consensus is available on the optimal amount of timing of TXA administration. Further research is needed to determine the most effective TXA regimen in patients who undergo surgical resection for extremity sarcomas.
ACKNOWLEDGMENTS
Open access funding provided by IReL.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
SYNOPSIS
Despite evidence of reduced blood loss, and lack of evidence of increased risk of venous thromboembolism events, the absence of prospective studies limits conclusive recommendations on TXA use in sarcoma surgery. Further research is warranted to determine optimal TXA regimens and assess safety concerns regarding thrombotic events in this patient population.




