Invasive squamous cell carcinoma of the breast associated with breast augmentation implant capsule
Abstract
Primary squamous cell carcinoma (SCC) of the breast is rare, representing less than 0.1% of all breast cancers. To date, there have been 20 reported cases of SCC associated with breast augmentation, usually in patients with long-standing implants. A patient is reported here with primary squamous carcinoma of the breast associated with textured saline implants. Due to the paucity of cases, there is limited information on the incidence and management of implant-associated SCC of the breast.
1 INTRODUCTION
Squamous cell carcinoma (SCC) of the breast is a rare histological subtype of breast cancer, representing less than 0.1% of all breast cancers.1 Strict diagnostic criteria are applied for primary SCC of the breast to exclude the possibility of spread from the skin or metastases from extramammary sites.2 To be classified as pure SCC of the breast, the tumor must be composed of >90% squamous cells, with no evidence of ductal or mesenchymal neoplasia.3 SCC of the breast has been described as an aggressive cancer, but due to the rarity of this disease, there are no established treatment guidelines.4, 5 These cancers have a variable response to chemotherapy, are refractory to treatment, and often have a poor prognosis.6, 7
It has been about 30 years since the initial report of a rare subset of breast SCC associated with long-standing breast augmentation implant capsules (BIA-SCC).8 Nearly all reported cases have occurred in patients who had breast augmentation over 10 years before presenting with unilateral painful breast swelling (Table 1).8-24 It has been shown in previous reports that BIA-SCC can occur with either silicone or saline implants, and that it can be an aggressive disease with poor prognostic implications.12, 15, 18, 19, 24 Here, a case of primary SCC of the breast is presented arising in a patient with long-standing saline implants.
First Author (Year) [Reference #] |
Age | Implant | Time | Operation | Pathologic findings | Overall survival | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
At SCC (years) |
Textured/ Smooth/Foam |
CONTENT Silicone/ Saline/NS |
Previous Revision |
Intact | Implant to SCC (years) |
Excision type | Axillary staging | Tumor size (cm) | Node mets | Tumor in capsule | SqEpi | Recurred < 12 mos. postop | |
| Paletta et al.8 | 52 | NS | Silicone | No | Yes | 15 | MR | Yes | 6 | No | Yes | Yes | No |
| Kitchen et al.9a | 42 | NS | Silicone | No | NS | 12 | C | No | 5.5 | NS | NS | No | NS |
| Satgunaseeian et al.10 | 58 | NS | NS | Yes | NS | 19 | TM | NS | NS | NS | Yes | Yes | NS |
| Zomerlei et al.11 | 58 | Smooth | Silicone | Yes | NS | NS | MR | Yes | 5.5 | No | Yes | Yes | NS |
| Olsen et al.12 | 56 | Text | Saline | Yes | Yes | 18 | MR | Yes | 3.5 | No | Yes | Yes | Yes |
| 81 | NS | Silicone | No | Yes | 42 | MR | Yes | 5 | No | Yes | Yes | Yes | |
| Buchanan et al.13 | 65 | Foam | Silicone | No | No | 21 | IR | NS | NS | Yes | NS | NS | No |
| Resetkova et al.14 | 41 | NS | Saline | NS | NS | 7 | MR | Yes | NS | NS | Yes | NS | NS |
| Zhou et al.15 | 46 | NS | Silicone | Yes | NS | 21 | C | NS | 4 | NS | NS | Yes | Yes |
| Alfaro et al.16 | 51 | NS | Saline | No | NS | 17 | NS | NS | NS | NS | Yes | Yes | NS |
| Goel et al.17 | 55 | NS | NS | Yes | NS | 34 | CWR | NS | NS | NS | NS | NS | NS |
| Zikiryakhodzhaev et al.18 | 49 | Text | Silicone | Yes | No | 19 | CWR | Yes | 4 | Yes | Yes | Yes | Yes |
| Goldberg et al.19 | 40 | Smooth | Saline | No | Yes | 11 | C | NS | NS | NS | Yes | Yes | Yes |
| 62 | NS | Silicone | Yes | Yes | 32 | C | NS | NS | NS | Yes | Yes | NS | |
| Liu et al.20 | 45 | NS | Silicone | No | NS | 10 | CWR | Yes | 6 | No | NS | NS | No |
| Soni et al.21 | 46 | Smooth | Saline | Yes | Yes | 6 | MR, C | Yes | 6 | No | Yes | Yes | No |
| Whaley et al.22 | 60 | Text | Saline | No | No | 27 | C | Yes | 3 | NS | Yes | Yes | No |
| 57 | NS | Saline | No | NS | 25 | C | NS | 7.5 | NS | Yes | Yes | NS | |
| Vorstenbosch et al.23 | 48 | NS | NS | NS | No | NS | CWR | NS | NS | NS | Yes | Yes | No |
| Xia et al.24 | 52 | Text | Silicone | No | Yes | 18 | C | No | 20 | No | Yes | Yes | Yes |
| Current case | 62 | Text | Saline | Yes | Yes | 15 | MR | Yes | 11.5 | No | Yes | Yes | Yes |
2 CASE REPORT
The patient was a 62-year-old postmenopausal Hispanic female, a former tobacco smoker, with a history of hypertension who underwent bilateral breast augmentation with subpectoral saline implants in 2008 at another facility. In 2010, she had a left implant rupture and underwent left breast augmentation revision with implant replacement using an Allergan BIOCELL® textured saline-filled implant (Style 168, Lot number 201-3112). The revision was complicated by an acute superficial surgical site infection treated with oral antibiotics.
In 2018, the patient reported being aware of a left breast mass, confirmed by mammogram. She was evaluated by a breast surgeon for tissue biopsy and potential implant removal but was lost to follow-up. In 2022, she noted worsening intermittent left breast pain associated with progressive swelling of the breast. In early 2023, the patient was referred by her primary care physician to our emergency department for evaluation of left breast mastitis after a 1-week history of acute exacerbation of left breast pain and swelling.
The patient did not have a history of nipple discharge. She was on medication for hypertension but took no other medications. The patient did not have a family history of breast, ovarian, or other cancers.
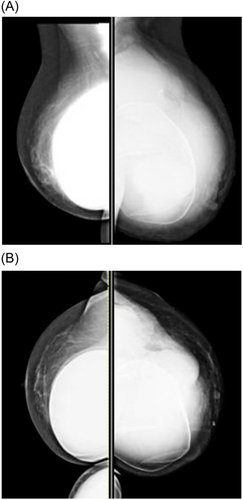
Physical examination of the left breast revealed a palpable, 5 cm. multiloculated tender mass at the 3 o'clock axis of the peri-areolar margin, within the previous implant incision (Figure 1A,B). The left breast was significantly enlarged, and there was central skin erythema and edema but no peripheral skin edema. The clinical findings were not consistent with a diagnosis of inflammatory cancer. There was no nipple discharge. There was no left axillary clinical lymphadenopathy. There were no suspicious physical findings in the previously implanted right breast or axilla. The patient was referred for diagnostic radiology work-up and was discharged on a short course of metronidazole, trimethoprim/sulfamethoxazole, and nasal mupirocin.

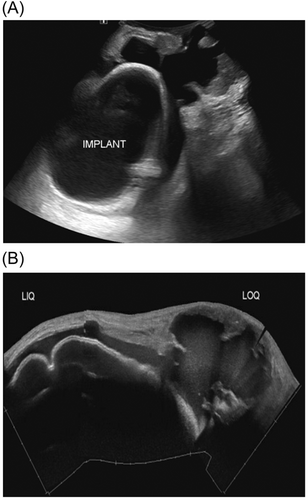
A diagnostic mammogram and ultrasound 5 days after the visit to the emergency department confirmed the presence of a BI-RADS 5 complex mass encompassing most of the left breast (Figure 2A,B). The mass measured 11 × 6 × 5 cm. Ultrasound demonstrated confluent peripheral irregular hypoechoic masses with a large fluid component surrounding an intact retropectoral implant, with invasion of the implant capsule (Figure 3A,B). A 12-gauge core needle biopsy of the solid tumor component was done at that time, and approximately 500 mL of yellow cloudy fluid was aspirated from the breast and sent for cytology and cultures. Ultrasonographic examination of the bilateral axillary lymph node basins revealed a suspicious right axillary node, for which a core biopsy was also done.
Pathologic examination of the core needle biopsy of the left peri-implant soft tissue mass revealed well-to moderately-differentiated invasive squamous carcinoma. The neoplastic cells were weakly positive for estrogen receptors in 60% of tumor cells (ER+) (60%), did not express progesterone receptors (PR-), and were negative for HER2 overexpression (HER2−). Stains for p63 and GATA-3 were positive. Cytology of the fluid revealed atypical squamous cells. Core biopsy of the right axillary lymph node revealed a reactive lymph node. Cultures were negative for growth of microorganisms.
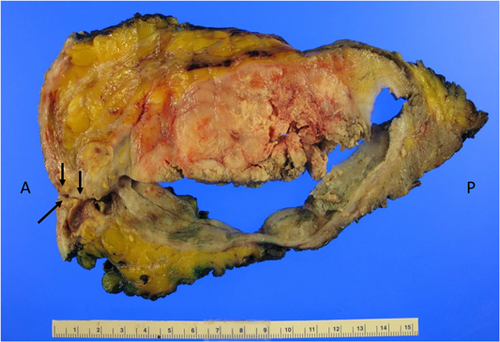
In late March 2023, the patient underwent left total mastectomy with complete capsulectomy and left axillary lymph node dissection. During the operation, tumor invasion into the left pectoralis major was encountered and a portion of the muscle was removed en bloc to ensure negative gross resection margins. Clear, yellow-tinged fluid was encountered during the dissection, emanating from the space between the implant capsule and the implant. After completion of the dissection, the specimen was opened for immediate postoperative gross examination. A 390 mL saline-filled implant was extracted and determined to be intact. Within the implant capsule, multiple loculated areas containing serosanguinous fluid and necrotic debris were identified. A 9.1 × 11.5 × 2.6 cm. friable, exophytic mass was identified, apparently arising from the implant capsule (Figure 4). A 4.5 cm. infra-areolar incision, previously used to place the implant in 2010, had an associated 0.5 cm. in diameter fistula tract communicating with the space between the implant capsule and the implant. The clinical impression at the operating table was that the fluid-filled space between the implant capsule and the implant represented necrotic tumor. The patient recovered from the operation without immediate complications, and she was discharged from the hospital on the first postoperative day.
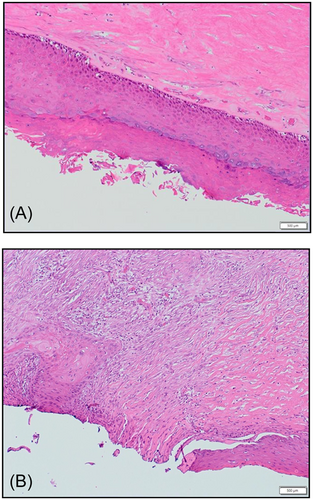
Microscopic examination of the mastectomy specimen demonstrated that the implant capsule was lined by keratinizing squamous epithelialization with areas of hyperkeratosis (Figure 5A). There was focal squamous dysplasia composed of increased basal mitoses with nuclear hyperchromasia and atypia, and a transition zone to invasive keratinizing, well-to moderately-differentiated SCC (Figure 5B). The tumor invaded through the capsule into the surrounding breast parenchyma. There was no evidence of atypia, in situ, or invasive duct or lobular carcinoma within the breast parenchyma. Surgical resection margins were free of invasive carcinoma. There was extensive giant cell reaction in breast parenchyma but no evidence of silicone-associated reaction in the breast or lymph nodes. Sixteen axillary lymph nodes were negative for metastatic carcinoma. Cytology of the intracapsular fluid revealed malignant squamous cells. The tumor hormone biomarkers were reconfirmed to be ER+ (60%), PR−, HER2−.
A postoperative positron emission tomography-computed tomographic scan did not demonstrate evidence of metabolically active metastatic disease. Recommendations for adjuvant therapy consisted of chemotherapy using carboplatin and paclitaxel followed by postmastectomy chest wall radiation. Within two months of the operation and after one dose of chemotherapy, the patient recurred in the left chest wall and supraclavicular fossa.
3 DISCUSSION
Breast implants have been widely used for aesthetic and reconstructive purposes since the 1970s in the United States. They continue to be one of the most common surgical procedures, with over 380 000 breast implant-related operations being performed every year.25 Large studies in the 1990s showed that both silicone and saline breast implants were safe and did not increase the risk of malignancy.26
In the early 2000s, clinicians started reporting on anaplastic large cell lymphomas (ALCL) arising within the implant capsule pocket.27, 28 By 2017, the United States Food and Drug Administration (FDA) had recorded 173 cases.29 These lymphomas were associated with textured breast implants, which were subsequently recalled in 2019. The implants used for the patient in this case report were among those recalled. Treatment for ALCL associated with breast implants (BIA-ALCL) consists of implant removal and total capsulectomy with metastatic workup.30 Prognosis is similar to that of patients with cutaneous ALCL and clearly better than that of patients with systemic ALCL.31 Complete capsulectomy has been associated with 5-year overall survival rates of 98% compared to 57% in patients not having complete capsulectomy. Favorable clinical-pathologic features associated with good prognosis include tumor confined to implant capsule at presentation and lack of regional lymph node involvement.
The FDA issued a new safety communication for breast implants on September 8, 2022, describing BIA-SCC and various lymphomas that were not associated with the previously reported BIA-ALCL.32 The earliest case reports of BIA-SCC were published in the 1990s.8, 9 As of March 22, 2023, the FDA UPDATE on BIA-SCC reported 19 reported cases.33 One of the two cases in reference 9 is a duplicate of the case in reference.8 Thus, with two additional case reports, there are 20 previously published cases at the time of this writing. (Table 1) Including the current case brings to 21 the total number of published cases. The median age of patients at the time of diagnosis was 52 years (range 40−81 years). Ten cases were associated with silicone implants, eight were associated with saline implants, and three were not specified. Five implants were textured, three were smooth, one was foam, and 12 were not specified. The median time from initial implant to diagnosis of BIA-SCC was 18 years (range approximately 6−42 years). Seven of the reported patients had recurrent cancer within 12 months after definitive resection.
Analysis of the 21 cases reveals some emerging themes regarding BIA-SCC. (1) Contrary to cases of BIA-ALCL for which there was an association between textured implants and lymphoma, there is not a consistent type (textured vs. smooth), content (silicone vs. saline), or location (subglandular vs. retropectoral) of implant that is associated with BIA-SCC. (2) The development of BIA-SCC typically occurs many years after placement of the original implant and is frequently associated with a history of prior revision(s) due to complications of the original augmentation. (3) The fibrous capsule is typically lined with benign or dysplastic squamous epithelium, and there is an identifiable transition between benign epithelium and invasive SCC. The location of the tumor may be either on the anterior (adjacent to breast parenchyma) or posterior capsule wall. (4) Recurrence of SCC in this series of patients appears to be a more common event than recurrence in patients with BIA-ALCL after capsulectomy, suggesting that BIA-SCC may be an aggressive form of implant-associated malignancy. Axillary lymph node involvement does not appear to be an obligatory harbinger of recurrent BIA-SCC.
Many authors reporting patients in this series have noted the relationship between recurrent or persistent abscesses within implant capsules, significant capsular contracture, the need for revision of the original breast augmentation, and the development of BIA-SCC. These observations have led to the postulation that inflammatory processes may be associated with the etiology of BIA-SCC. It is not currently known how inflammatory processes might be related to the uniform findings in these cases that benign squamous epithelium lined the fibrous implant capsules and that BIA-SCC arose from benign or dysplastic squamous epithelium in the implant capsules. It is interesting to note that in the current case, there was a fistula lined by squamous epithelium between the periareolar incision used to replace the previous implant and the implant capsule containing the invasive SCC. It is possible that either the squamous epithelial lining of the implant capsule occurs from de novo development of squamous metaplasia from the mesodermal layer due to chronic inflammation, or that direct squamous epithelial cell seeding of the implant capsule occurs as a result of drainage or revision procedures.19 It has also been proposed that during breast augmentation, mammary ducts are transected, and that there may be subsequent squamous metaplasia of endoderm-derived duct epithelium, as is a common reaction to chronic inflammation in other parts of the body.13 SCC arising from areas of chronic inflammation are more familiar as Marjolin's ulcers, developing from skin in an area exposed to chronic irritation or from sinus tracts draining chronic osteomyelitis.
Similar to the case presented here, all previously published cases were detected not by routine breast screening but with an initial presentation of breast pain, erythema, and swelling in the presence of a breast implant. As the differential diagnosis for a complex cystic and solid mass involving a breast implant includes BIA-ALCL, NCCN guidelines for BIA-ALCL are useful to arrive at a diagnosis.34 Breast magnetic resonance imaging helps to define the relationship between tumor and loco-regional anatomy and to assess for resectability. If a mass is identified, core needle biopsy is recommended. Periprosthetic fluid collections should be aspirated under ultrasound guidance and evaluated for cytology. For BIA-ALCL, immunohistochemistry and/or flow cytometry may include evaluation for CD2-5, CD7, CD8, CD30, CD45, and ALK.34 For BIA-SCC, fluid should be sent for CK5/6 and p63. In patients with BIA-SCC, aspirated fluid should be rich in keratin, and cytology should display abnormal squamous cells.23
Various adjuvant treatments for BIA-SCC, including chemotherapy and radiation, have had mixed results. Previously reported chemotherapy regimens have generally followed those used for primary SCC of the breast. Despite aggressive treatment, the prognosis has been poor in some of the previously reported patients. Because of the paucity of experience with BIA-SCC, there are no current recommendations for treatment and management of this condition.
4 CONCLUSIONS
This case report highlights the need to raise awareness regarding the existence of SCC of the breast in the Plastic Reconstructive Surgery and Breast Surgical Oncology communities, emphasizing that ALCL is not the only malignancy associated with breast implants. The American Society of Plastic Surgery and International Society of Aesthetic Plastic Surgeons currently encourage physicians to continue with routine clinical follow-up of breast implants and to report any events to the FDA and national professional organizations.35
ACKNOWLEDGMENTS
Translation of reference18 from Russian to English courtesy of Yuliya Olimpiadi, MD. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Open Research
DATA AVAILABILITY STATEMENT
Data used in this study are available from the corresponding author upon reasonable request.
REFERENCES
SYNOPSIS
Although it is a rare event, this case report emphasizes that surgeons should be aware of the association of invasive squamous cell carcinoma with breast augmentation implant capsules.




