The Use of Transporter Probe Drug Cocktails for the Assessment of Transporter-Based Drug–Drug Interactions in a Clinical Setting—Proposal of a Four Component Transporter Cocktail
Abstract
Probe drug cocktails are used clinically to assess the potential for drug–drug interactions (DDIs), and in particular, DDIs resulting from coadministration of substrates and inhibitors of cytochrome P450 enzymes. However, a probe drug cocktail has not been identified to assess DDIs involving inhibition of drug transporters. We propose a cocktail consisting of the following substrates to explore the potential for DDIs caused by inhibition of key transporters: digoxin (P-glycoprotein, P-gp), rosuvastatin (breast cancer resistance protein, BCRP; organic anion transporting polypeptides, OATP), metformin (organic cation transporter, OCT; multidrug and toxin extrusion transporters, MATE), and furosemide (organic anion transporter, OAT). Furosemide was evaluated in vitro, and is a substrate of OAT1 and OAT3, with Km values of 38.9 and 21.5 μM, respectively. Furosemide was also identified as a substrate of BCRP, OATP1B1, and OATP1B3. Furosemide inhibited BCRP (50% inhibition of drug transport: 170 μM), but did not inhibit OATP1B1, OATP1B3, OCT2, MATE1, and MATE2-K at concentrations below 300 μM, and P-gp at concentrations below 2000 μM. Conservative approaches for the estimation of the likelihood of in vivo DDIs indicate a remote chance of in vivo transporter inhibition by these probe drugs when administered at low single oral doses. This four component probe drug cocktail is therefore proposed for clinical evaluation. © 2015 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:3220–3228, 2015
INTRODUCTION
Transporters play a critical role in facilitating the absorption, distribution, and elimination of many drugs. It is recognized that nearly all drugs and metabolites of drugs are likely to interact to a certain extent with membrane transporters.1, 2 Therefore, determining the potential for interaction of new drug candidates with drug transporters including ATP-binding cassette (ABC) and solute carrier (SLC) transporters has become an indispensable part of nonclinical and clinical drug development. The important role of transporter-based drug–drug interactions (DDIs) has consequently resulted in the inclusion of this topic in recent regulatory guidelines.3-5 Generally, such investigations start with in vitro studies and result in data that, when considered together with pharmacokinetic parameters for a drug candidate, can be used to make an informed decision regarding the assessment of transporter-based DDIs in dedicated clinical trials.
Although drugs in development are routinely evaluated for their potential interactions with a variety of transporters, the results of such in vitro transporter investigations have to be interpreted carefully because of the lack of highly specific tools and the use of complex experimental systems.1 In addition, drug transporters generally exhibit broad substrate specificity, and it is frequently observed that drugs interacting with one transporter will most likely interact with many other transporters.2, 6 Contributing to this challenging situation is the fact that results generated using different experimental systems in different laboratories are highly variable.7 The lack of definitive and standardized in vitro methodologies for reliably predicting clinical transporter-mediated DDIs has been recognized by regulatory agencies, who have in response taken a cautious position when evaluating in vitro transporter data within the context of patient safety. As a consequence, the need for an increased number of clinical drug transporter-based DDI trials may be triggered by this cautious interpretation of in vitro data. In addition, because of the broad substrate specificity of drug transporters, a single clinical DDI trial may not be sufficient to explore the entire “interaction potential” between a drug and a transporter.
In order to effectively explore whether a drug candidate will act as an inhibitor of several drug transporters in vivo, a probe drug cocktail approach may be applied to investigate potential clinical DDIs. Such an approach is based on the simultaneous dosing of a group of drugs as probe substrates for the most important transporters from a DDI perspective, together with the drug candidate being investigated, followed by an assessment of effects of the drug candidate on the pharmacokinetic profiles of the probe drugs. Various probe drug cocktails, such as the “Pittsburgh Cocktail” or “Cooperstown Cocktail” have been utilized in drug development for the assessment of DDIs that are based on interactions with drug metabolizing enzymes, specifically cytochrome P450 (CYP450) enzymes.8-10 On the basis of this precedent and their successful use over the last years, guidelines established by the European Medicines Agency (EMA), US Food and Drug Administration (FDA), and Pharmaceuticals Medical Devices Agency (PMDA) explicitly make reference to the use of probe drug cocktails to perform clinical DDI trials.
Presently, there is no published literature describing the use of dedicated transporter probe drug cocktails, and according to currently available information in the public domain, no such trials have been performed. To explore the possibility of developing a cocktail-based approach to evaluate transporter-mediated DDIs, in this paper, we propose the use of a four-component probe drug cocktail and support our concept with in vitro data that indicate the suitability of the chosen probe drugs. Additionally, as adequate literature data for one of the drugs we propose to include in the probe drug cocktail, furosemide, was not available, we conducted a series of in vitro studies to characterize the potential for furosemide to interact with key ABC and SLC transporters.
MATERIALS AND METHODS
Chemicals
Acylovir, cimetidine, digoxin, estrone 3-sulfate (E-sul), fumitremorgin C (FTC), furosemide, 1-methyl-4-phenylpyridinium (MPP+), and rifampicin were obtained from Sigma–Aldrich (St. Louis, MO, USA) . Metformin and probenecid were obtained from Wako (Osaka, Japan). The multidrug resistance-associated protein (MRP) inhibitor MK-571, the MATE inhibitor pyrimethamine, and furosemide d5 (furfuryl-d5) were obtained from Alexis Biochemicals (Lausen, Switzerland), MP Biomedicals (Santa Ana, CA, USA), and CDN Isotope (Quebec, Canada), respectively. [3H]Digoxin and [3H]E-sul were obtained from PerkinElmer (Waltham, MA, USA). [3H]Acylovir and [14C]mannitol were obtained from Moravek Biochemicals, Inc. (Brea, CA, USA). [3H]Rosuvastain, [14C]metformin, [3H]MPP+, and [3H]metoprolol were from obtained American Radiolabeled Chemicals, Inc. (St. Louis, MO, USA). Zosuquidar (ZSQ) was prepared by custom synthesis. All other chemicals were of the highest reagent grade available from commercial sources.
Plasmids
Plasmids (pcDNA5/FRT-DEST; Invitrogen) were used that contain cDNA of organic anion transporter (OAT) 1, OAT3, organic anion transporting polypeptide (OATP) 1B1, OATP1B3, or organic cation transporter (OCT) 2.11, 12
Cells
Caco-2 cells were purchased from the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). Parental HEK293 cells were purchased from Health Science Research Resource Bank (Japan). HEK293 cells stably transfected with either empty vector, multidrug toxin extrusion (MATE) 1, or MATE2-K were obtained from GenoMembrane, Inc. (Japan) under license agreement.
Cell Culture
Caco-2 cells were maintained as described in a previous report.13 About 1 × 106 cells were seeded per flask. The culture medium was changed three times each week. Caco-2 cells were seeded at a density of 1.5 × 105 cells/cm2 on Transwell filter inserts. The cells were cultured at 37°C, 8% CO2, and 90% relative humidity in Dulbecco's modified Eagle medium (DMEM) culture medium for 15–16 days. Parental HEK293 cells were maintained in DMEM culture medium [low-glucose DMEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS; Invitrogen), 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 g/mL amphotericin B] at 37°C, 5% CO2, and 95% relative humidity. HEK293 cells stably transfected with empty vector; MATE1 or MATE2-K were maintained in DMEM culture medium supplement with 10% FBS, 50 U penicillin, 50 μg/mL streptomycin, and 500 μg/mL G418 (Invitrogen). The transport activity of each cell line was confirmed by examining the uptake of probe substrates.
Cellular Uptake
For expression of OAT, OATP, and OCT transporter isoforms, parental HEK293 cells were seeded onto lysine-coated 24-well plates at a density of 0.75 × 105 cells/well. On the next day, transfection was conducted according to manufacturer's protocol. Approximately 24 h after transfection, culture medium was changed to DMEM culture medium supplemented with 5 mM sodium butyrate, and incubated in culture medium for an additional 24 h to allow for plasmid transporter gene expression. For MATE1 and MATE2-K, HEK293 cells stably expressing MATE1 or MATE2-K, or stably transfected with empty vector, were seeded onto lysine-coated 24-well plates at a density of 1.5 × 105 cells/well and were cultured for 3 days. On the day of experiment, cells expressing transporter isoform transiently were rinsed twice and preincubated in transport buffer (modified Krebs–Henseleit buffer supplemented with 25 mM HEPES, 1.5 mM calcium chloride) at 37°C for 15–60 min. Stably transporter-expressing cells were rinsed twice with MATE transport buffer (130 mM KCl, 2 mM KH2PO4, 1.2 mM MgSO4 7H2O, 1 mM CaCl2 2H2O, 20 mM HEPES, 5 mM d-(+)-glucose, pH 7.4) and the cells were preacidified with MATE transport buffer supplemented with NH4Cl (final 20 mM) for 10 min at 37°C. The MATE transport buffer with NH4Cl was replaced with normal MATE transport buffer and cells were incubated for additional 5 min. For both transient and stable systems, the transport buffer was removed and replaced with fresh transport buffer containing radiolabeled drug and varying concentrations of inhibitor specific for each transporter. Initial drug concentration was determined at the initiation of the incubation (t0). The concentration of the solvent in which the drug was initially dissolved did not exceed 0.5% by volume. The incubation was stopped by aspirating the buffer and replacing it with ice-cold transport buffer. The cells were rinsed three times with ice-cold transport buffer, and the buffer was aspirated after the final rinse. For radioactivity measurement, the cells were lysed with NaOH for 1 h at 37°C. The reaction was neutralized with HCl. Radioactivity in the cells was determined for 3 min in a liquid scintillation analyzer (TRI-CARB 3100TR and 3110TR; Packard). Protein concentrations were measured using the Lowry method. For furosemide measurement, cells were collected using a rubber scraper after the addition of 200 μL water. Adequate amounts of cell suspension were transferred to test tubes and the volume was adjusted to 180 μL with blank matrix that was prepared using untreated cells on the same day of experiment.
Transcellular Transport Assay
Transcellular transport experiments were performed as triplicate incubations using different filter inserts for both the AtoB and BtoA direction. Cells were equilibrated in Hank's buffered saline solution (HBSS) transport buffer [pH 7.4, 15 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid] for 30 min. The donor side of the cell monolayer was filled with transport buffer containing the substrate with or without inhibitor. The receiver chamber was filled with transport buffer with or without inhibitor. Assays were started after a preincubation period of 30 min. Samples were taken at −30, 0, and 90 min from the donor compartment, representing the actual start of equilibrium, end of equilibrium, and end concentrations in the donor compartment. Samples were also taken at 0, 30, 60, and 90 min from the receiver compartment. Sample aliquots withdrawn at each time point from the receiver compartment were immediately replaced with an equal volume of fresh receiver solution. All samples collected were transferred into 96-well plate. Radioactivity was measured by TopCount (PerkinElmer) after complete dry up at 50°C overnight. For furosemide, volume of samples collected was adjusted to 180 μL with HBSS transport buffer for liquid chromatography–mass spectrometry (LC–MS/MS) analysis.
LC–MS/MS Analysis for Furosemide
Furosemide concentrations in all samples from cellular uptake and from transcellular transport assays were quantified using a LC–MS/MS system consisting of a API 4000TM triple quadruple mass spectrometer (AB Sciex, Foster City, California) coupled with a Agilent 1100 HPLC system (Agilent Technologies, Santa Clara, California). LC–MS/MS analysis was performed under the conditions given below. A Hypersil GOLD C18 analytical column (2.1 × 50 mm2, 5 μm) was used as the analytical column. The mobile phase contained 0.1% formic acid in acetonitrile and 0.1% formic acid in water (50:50, v/v), with a flow rate of 0.3 mL/min and a run time of 5 min. Furosemide and Furosemide-d5 were detected by performing negative-ion electrospray tandem mass spectrometry at −4500 V and 500°C, with the mass transitions of m/z 329–205, and 334.1–205.8, respectively, based on a calibration curve constructed by the analysis of 0.5–200 pmol/180 μL authentic standard solution for cellular transport assay, and 1–200 pmol/180 μL for donor and receiver solution for transcellular transport assay. Analyst software version 1.5.1 (AB Sciex) was used for data analysis.
Calculations
Apparent Permeability Coefficient
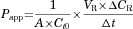
Efflux Ratio
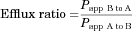
Cellular Uptake
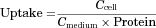
Transporter-mediated uptake was calculated by subtracting the uptake in vector transfected cells from that in transporter expressing cells.
50% Inhibition of Drug Transport
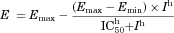
RESULTS
Inhibitory Effect of Digoxin, Furosemide, Metformin, and Rosuvastatin on Target Transporters
The potential inhibitory effect of the four drugs was investigated using Caco-2 cells for P-gp and BCRP, and transfected HEK293 cells for OAT1, OAT3, OATP1B1, OATP1B3, OCT2, MATE1, and MATE2-K (Table 1). DDI likelihood was evaluated based on IC50 values and various parameters of drug concentration at the DDI site using the decision trees recommended by the FDA and EMA.3, 4 DDI assessment ([I]1,total/IC50 for systemic P-gp and BCRP, [I]1, unbound/IC50 for renal transporters, [I]in,max, u/IC50 for hepatic uptake transporters and [I]2/IC50 for intestinal efflux transporters) revealed that mutual DDI among the four drugs is unlikely to occur except for rosuvastatin inhibition of OATP1B1 ([I]in max,u/IC50 of rosuvastatin against OATP1B1 = 0.14–0.2, which is higher than the EMA cutoff criteria, 0.04, but lower than the FDA cutoff criteria of 0.25). However, by taking higher cut-off criteria into consideration from both agencies, it is regarded as unlikely that rosuvastatin will act as a perpetrator of DDIs by inhibiting OATP1B1 transporter activity.3, 4
| In Vitro Inhibitory Effect, IC50 (μM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| P-gp | BCRP | OAT1 | OAT3 | OATP1B1 | OATP1B3 | OCT2 | MATE1 | MATE2-K | |
| Digoxin | 12514 | >30 | >30 | >30 | 7.915–4716 | 1–4215-17 | >30 | >30 | >30 |
| Furosemide | >2000 | 170 | 5.118–2019 | 1.720–5118 | 30–300 | >300 | >300 | >300 | >300 |
| Rosuvastatin | >400 | 140 | >100 | 2621 | 0.04722–2.4323 | 3.6123 | >100 | >100 | >100 |
| Metformin | >30,000 | >30,000 | >1000 | >1000 | >1000 | >1000 | 28924–170025 | 66726 | 650026 |
| In Vivo Concentration at DDI Site | |||||||||
| Dose (mg) | [I]1,total (μM) | [I]1,unbound (μM) | [I]in,max,u (μM) | [I]2 (μM) | DDI Likelihood3, 4 | ||||
| Digoxin | 0.25 | 0.0026a 27 | 0.0019 | 0.018 | 1.3 | Remote | |||
| Furosemide | 5 | 0.42b,28 0.64b 29 | 0.0059, 0.0090 | 0.020, 0.023 | 60 | Remote | |||
| Rosuvastatin | 10 | 0.009530 | 0.0011 | 0.0066–0.010 | 83 | Remote | |||
| Metformin | 500 | 1031 | 10 | 210 | 12,000 | Remote | |||
- a Based on maximum therapeutic serum concentrations of 2 ng/mL.
- b [I]1,total was calculated assuming dose proportionality up to a 40-mg oral dose.
- [I]1, unbound (μM) was calculated using [I]1 total and unbound fraction (Table 2). [I]in, max, u (μM) equals fu × ([I]1, total + (ka × dose × FaFg/Qh), where ka, FaFg, and hepatic blood flow was set as 0.1 min−1, 1 and 1500 mL/min, respectively, for furosemide, metformin, and digoxin. The FaFg and ka of rosuvastatin used was 0.4332 and 0.46–0.78 (1/h),33 respectively. [I]2: Dose (mol)/250 mL. The probe substrates used as P-gp, BCRP, OAT1, OAT3, OATP1B1/1B3, and OCT2 are digoxin (1 μM), E-sul (10 μM), acyclovir (0.05 μM), E-sul (0.1 μM), rosuvastatin (0.1 μM), and metformin (10 μM), respectively.
Involvement of ABC Transporters in Furosemide Transport
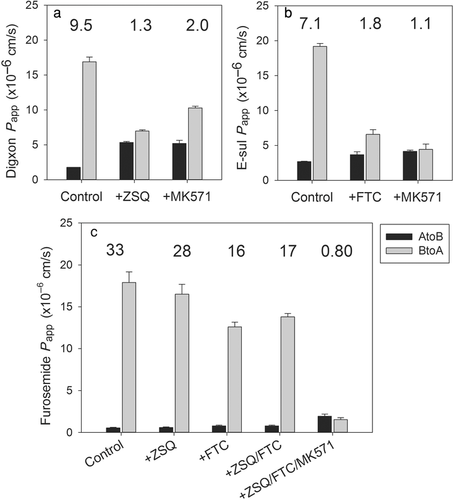
There is limited published information available concerning the active transport of furosemide and its suitability as an in vivo probe substrate to evaluate interactions with OAT1 and OAT3. This is in contrast to digoxin, metformin, and rosuvastatin, all of which have been frequently used as in vivo probes for P-gp, OCT/MATEs, and OATPs, respectively (Table 2). Therefore, in vitro substrate profiling of furosemide was performed to assess the suitability of furosemide for use in the transporter cocktail. The involvement of ABC transporters such as P-gp and BCRP was assessed using Caco-2 cells, as Caco-2 cells express functional P-gp, BCRP, and MRP2.34 It has been reported that ZSQ and FTC can selectively and effectively inhibit P-gp and BCRP at relatively low concentrations (1–3 μM), and MK-571 is a nonselective MRP inhibitor but can be used as a suitable MRP inhibitor if it is included as one component of the triple combination of ZSQ, FTC, and MK-571 in the Caco-2 cell system.35 Furthermore, the tiered approach is proposed to evaluate the involvement of P-gp, BCRP, and other efflux transporters such as MRP using Caco-2 cells and optimized concentrations of ZSQ (3 μM) and FTC (1 μM) and the triple combination of ZSQ, FTC, and MK-571.35 The selectivity profile of ZSQ, FTC, and MK-571 against P-gp, BCRP, and potentially MRP was investigated in the Caco-2 cells used in this study (Fig. 1). It was confirmed that ZSQ (1 μM) and FTC (1 μM) are selective P-gp and BCRP inhibitors, respectively, and MK-571 (50 μM) is a nonselective inhibitor of P-gp and BCRP.13 Using these three chemical inhibitors, the contribution of P-gp, BCRP, and other transporters that potentially could be inhibited by MK-571 was evaluated. The transcellular transport of furosemide in the absence of inhibitors (ER: 33) did not substantially change in the presence of ZSQ (ER: 28); however, there was a substantial difference when furosemide was incubated with FTC (ER: 16). Coincubation with ZSQ and FTC did not result in a significant change (ER: 17 at ZSQ/FTC). In contrast, coincubation of ZSQ, FTC, and MK-571 diminished the vectorial transport of furosemide to an ER value of 0.8. This indicates that transporters sensitive to inhibition by FTC (BCRP) and MK-571 (probably MRP2) drive the active efflux of furosemide in Caco-2 cells.
| Digoxin27 | Rosuvastatin36 | Furosemide37 | Metformin38 | |
|---|---|---|---|---|
| BDDCSa 39 | 3 | 3 | 4 | 3 |
| Mode of Action | Sodium–Potassium ATPase Inhibitor | HMG CoA Reductase Inhibitor | Sodium/Chloride Reabsorption Inhibitor | Increasing Peripheral Glucose Uptake and Utilization |
| Clinical dose range (mg/day)b | 0.125–0.25 (0.5) | 5–20 (40) | 40–80 | 850–2250 |
| Proposed dose in cocktail (mg) | 0.25 | 10 | 5 | 500 |
| Half-life (h) | 39 | 20 | 1.3 (0.5–2.4) | 1.74 (1.5–4.5) |
| Oral bioavailability (%) | 70 | 20 | 61 | 52 |
| Unbound fraction (%) | 75 | 12 | 1.4 | 99.9 |
| Route of elimination | i.v. Dosing: 51% urine, 15% faecesc 40 | i.v. Dosing: 28% urine; p.o. dosing: 90% faeces | i.v. Dosing: 83% urine, 7.5% faeces; p.o. dosing: 55% urine, 35% faeces41, 42 | i.v. Dosing: 100% urine; p.o. dosing: 52% urine, 29% faeces42 |
| Ratio of CLr/GFR | 1.24 after i.v.43 | 20 after i.v.44 | 37–158 after i.v.45 | About 3.5 after i.v. |
| Metabolism | Minor | Minor (∼ 20%) | Minor | Negligible |
| In vitro transporterd | P-gp, OATP1B3,16, 46, 47 OCT248 | P-gp,49 BCRP,50 OAT3,21 OATP1B1,50 OATP1B350 | BCRP,51 OAT1,52 OAT352 | OCT2,53 MATE1,53 MATE2-K,53 P-gp,54 BCRP54 |
| Used as clinical probe substrate | Yes55 | Yes56, 57 | No | Yes31, 58 |
Involvement of SLC Transporters in Furosemide Transport
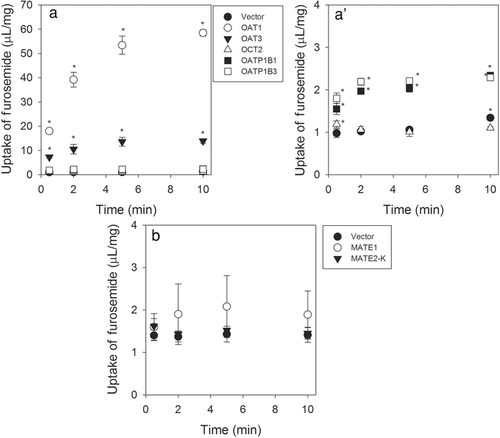
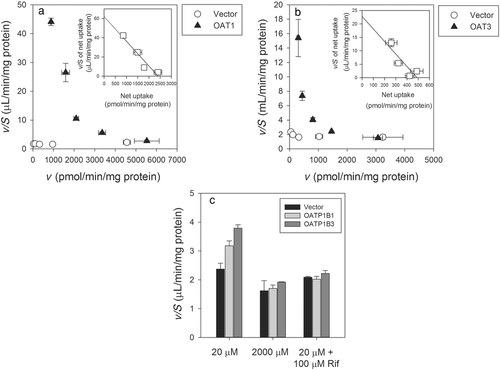
Using HEK293 cell expressing OAT1, OAT3, OATP1B1, OATP1B3, OCT2, MATE1, or MATE2-K, the involvement of SLC transporters in the active uptake of furosemide was evaluated. Uptake of furosemide (20 μM, a concentration selected because of sensitivity limits of the analytical assay) was significantly higher in OAT1-, OAT3-, OATP1B1-, or OATP1B3-expressing HEK293 cells compared with that observed in the vector-transfected cells (Fig. 2). In contrast, cells expressing the other transporters evaluated did not consistently demonstrate significantly higher uptake of furosemide compared with vector-transfected cells (Fig. 2). In order to confirm results from the time course assay, concentration-dependent transport was assessed using furosemide concentration range of 20–2000 μM (Fig. 3). OAT1 and OAT3 showed clear concentration-dependent saturation of furosemide uptake, and calculated Km values for OAT1 and OAT3 from Eadie–Hofstee plots were 38.9 and 21.5 μM, respectively (Fig. 3). In experiments involving OATP transporters, Km values for furosemide could not be accurately calculated, because the concentration dependency was not definitively demonstrated when compared with that seen OAT1- and OAT3-expressing cells. However, concentration-dependent reduction of uptake clearance and complete inhibition of furosemide uptake by the typical OATP inhibitor rifampicin was observed. These results clearly suggest that furosemide is a substrate of OAT1, OAT3, OATP1B1, and OATP1B3 but not a substrate of OCT2, MATE1, and MATE2-K.
DISCUSSION
The field of drug transporters presents inherently difficult challenges, such as the fact that very few selective substrates and inhibitors have been identified for clinical use, especially compared with what is known regarding CYP450-mediated DDIs. Additionally, relatively sparse conclusive clinical data is available describing transporter-mediated DDIs. Therefore, probe drugs were considered for which in vitro and in vivo information was available describing their interaction with the key drug transporters, followed by an assessment to determine their potential to undergo clinically relevant transporter-based DDIs. Importantly, probe drugs were selected not only to identify those that are expected to be most sensitive to inhibition by coadministered drugs, but also to exclude the potential for mutual interactions between the various selected components of the cocktail. In addition, clinical safety and usability as well as commercial availability for the combination of drugs selected were carefully considered. Of the numerous potentially useful probe drugs that were evaluated, rosuvastatin, metformin, and digoxin were selected as probes of OATPs/BCRP, OCT/MATEs, and P-gp, respectively.
Furosemide was chosen as an OAT probe drug as other alternatives were not easily accessible in different countries (e.g., cephradine) or of concern with respect to safety in healthy volunteer studies (e.g., tenofovir). Therefore, a low dose of 5 mg of furosemide was selected as probe for OAT-based DDIs, and this low dose can be orally administered using the commercially available formulation that is typically used for intravenous administration. By selecting a low dose of 5 mg, the diuretic effect of a standard 40 or 80 mg therapeutic dose of furosemide will be greatly diminished, while preserving the ability to detect changes in furosemide exposure using LC–MS/MS analysis. Furosemide was shown not to be a potent inhibitor of ABC and SLC transporters, based on criteria listed in guidelines from regulatory authorities. In our studies, furosemide was found to be a good substrate of OAT1 and OAT3, and also a substrate of OATP1B1 and OATP1B3 (Fig. 3). The uptake clearance of furosemide at 20 μM, the lowest substrate concentration, was much higher in cells expressing OAT3 and OAT1 (>20 μL/2 min/mg protein) compared with its uptake clearance in cells expressing in OATP1B1 and OATP1B3 (<2.5 μL/2 min/mg protein) (Fig. 3). This difference in uptake clearance obtained from in vitro systems most likely explains why furosemide is mainly excreted into urine, that is, more than 80% of the administered i.v. dose, suggesting that renal clearance is mainly mediated by active secretion (Table 2).41 To determine the relative contribution of OAT1 and OAT3 at basolateral transport of proximal tubule cells, protein expression data in human proximal tubule cells and in recombinant systems is essential as mRNA is only a surrogate marker of protein and discrepancy between mRNA and protein expression of transporter has been reported.59 However, at present, data describing the quantitative expression of OAT1 and OAT3 in human proximal tubule does not exist, and therefore determination of the relative contribution of these transporters to active uptake of substrates such as furosemide into renal cells is not possible based on the in vitro data obtained in this study. Transport experiments in Caco-2 monolayers indicated that furosemide is a substrate of transporters sensitive to inhibition by MK-571, possibly MRP2. Contribution of MRP4 to furosemide transport has been reported.60 Because of the relatively broad substrate specificity of drug transporters, it is possible that, although a drug may interact with several membrane transporters, this interaction may not necessarily result in a clinically relevant effect on its pharmacokinetic profile. Similar to OAT1 and OAT3, quantitation of MRP2 and MRP4 protein expression in renal proximal tubules has not been reported; thus, the relative contribution of these transporters toward the renal clearance of furosemide into urine cannot be accurately assessed. Additionally, there are very few DDIs that have been ascribed to inhibition of MRP2 and MRP4, and apart from cyclosporine A, clinically usable drugs that function as inhibitors of MRP2 and MRP4 have not been identified.2 The potential for transporter-mediated DDIs could depend on transporter expression levels in not only kidney but other organs as well, and is also a function of the relative drug–transporter interaction based on the kinetics of affinity and rate of transport. In this paper, our focus is on drug transporters recognized for their relevance on drug safety and efficacy with an emphasis on the potential for discernable changes in pharmacokinetics resulting in clinically relevant DDIs.2-5
The aforementioned lack of safe and selective substrates and inhibitors of transporters for in vitro and clinical studies is an ongoing challenge, and is one that is not confined to P-gp, BCRP, and the OATP and OAT families of transporters. Our in vitro data confirm that this phenomenon represents a consideration that applies to many drugs that would otherwise be considered suitable for use as components in a clinical transporter DDI probe drug cocktail. Nevertheless, a cocktail that lacks the single-enzyme specificity of cytochrome P450 probe drugs would still be highly valuable for the assessment of transporter-based DDIs. The selected probe drugs will address key processes of transporter-mediated drug disposition and elimination, that is, hepatic uptake by OATPs, renal elimination by OCTs, MATE, and OATs, and intestinal secretion by P-gp and BCRP, and thereby provide a better understanding of principles of DDIs that are mediated by drug candidates. Data from an initial transporter probe drug cocktail study can then serve as a guide for focused analysis and possible follow-up studies with selected probe drugs to address the identified physiological processes affected by transporter DDIs. A successful cocktail DDI trial design will also help to assess the validity of in vitro transporter inhibition data generated in support of drug candidates, with respect to relevance and predictive value in an in vivo situation.
The use of this probe drug cocktail for clinical assessment of DDIs, however, requires validation in a clinical setting. Proposed doses of these probe drugs, their clinical pharmacokinetic profiles, and available transporter data are shown in Table 2. Pharmacokinetic trials in healthy subjects are needed to exclude mutual interactions of the cocktail components, to investigate the effects of known prototypical inhibitors, and to assess safety and practical applicability of the proposed cocktail. Clinical studies to assess these issues for the proposed four component probe drug cocktail are currently ongoing.
CONCLUSION
To assess the processes of drug disposition and elimination, and considering the number of transporters that are mandatory for assessment in drug development, we propose a four component probe drug cocktail comprising digoxin, furosemide, metformin, and rosuvastatin.3-5 The proposed four component probe drug cocktail is based on a literature survey of published in vitro data, previously unavailable in vitro data obtained for furosemide from our in vitro studies, and results of published clinical trials on pharmacokinetics and DDIs studies involving the proposed probe drugs. As safety was a principal consideration in our selection process, the proposed cocktail uses low doses that are considered to be safe when administered using a single dose regimen. In addition, probe drugs were selected that are registered and readily available for use in many countries.
ACKNOWLEDGMENTS
The excellent technical assistance of Ikumi Washio, Asami Saito, Naoko Otsu, Hidetada Shimizu, Tokuko Takatsuka, Christian Lechner, Ayano Fukuhara, Junichi Takano, and Masahito Takatani in conducting the in vitro experiments at Nippon Boehringer Ingelheim is gratefully acknowledged.
Special thanks are given to the members of the “Transporter Cocktail Team” at Boehringer Ingelheim: Thomas Giessmann, James Hilbert, Fabian Müller, Ashish Sharma, Peter Stopfer, and Heike Zimdahl-Gelling.
The authors wish to thank Martin Fromm, Uwe Fuhr, Kathleen Giacomini, Richard Kim, Mikko Niemi, and Peter Swaan for their input to this project.




