Two-Stage Single-Compartment Models to Evaluate Dissolution in the Lower Intestine
Abstract
The purpose was to propose two-stage single-compartment models for evaluating dissolution characteristics in distal ileum and ascending colon, under conditions simulating the bioavailability and bioequivalence studies in fasted and fed state by using the mini-paddle and the compendial flow-through apparatus (closed-loop mode). Immediate release products of two highly dosed active pharmaceutical ingredients (APIs), sulfasalazine and L-870,810, and one mesalamine colon targeting product were used for evaluating their usefulness. Change of medium composition simulating the conditions in distal ileum (SIFileum) to a medium simulating the conditions in ascending colon in fasted state and in fed state was achieved by adding an appropriate solution in SIFileum. Data with immediate release products suggest that dissolution in lower intestine is substantially different than in upper intestine and is affected by regional pH differences > type/intensity of fluid convection > differences in concentration of other luminal components. Asacol® (400 mg/tab) was more sensitive to type/intensity of fluid convection. In all the cases, data were in line with available human data. Two-stage single-compartment models may be useful for the evaluation of dissolution in lower intestine. The impact of type/intensity of fluid convection and viscosity of media on luminal performance of other APIs and drug products requires further exploration. © 2015 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:2986–2997, 2015
INTRODUCTION
Dissolution in the lower intestine is of interest in cases of orally administered active pharmaceutical ingredients (APIs) with extended and/or delayed absorption kinetics. Relevant scenarios include immediate release products of highly dosed APIs and modified release drug products.
For evaluation of colonic absorption, knowledge of drug solubility and dissolution rates in the region is required but relevant estimations remain problematic because of limited information on the conditions prevailing in the lower intestine.1 In recent years, our understanding on the environment in the lower intestine has been increased.2-5 Solubility of lipophilic APIs in the lumen of the ascending colon seems to be different than in the contents of stomach and/or upper intestine during fasting state.6 In the fed state, although solubility in the ascending colon seems to be lower compared with the fasted state, data to date indicate that it is much higher than in simple aqueous media.6 Proposals for simulating the composition of contents in the lower intestine have already appeared in the literature.6, 7
In vitro evaluation of dissolution from modified release products into the lumen of lower intestine is addressed by using various, typically multicompartmental, setups and focusing to the robustness of dosage form throughout the gastrointestinal (GI) lumen8, 9 but, also, by using single-compartment setups focusing on the impact of luminal composition and mechanical stresses on the dosage form performance.10
The objective of the present investigation was to propose in vitro two-stage single-compartment models for evaluating dissolution characteristics in the lower intestine, that is, distal ileum and proximal colon, under conditions simulating the bioavailability and bioequivalence studies in the fasted and in the fed states. To evaluate the importance of simulating certain luminal characteristics, within a specific region of intestinal lumen two levels of simulation of luminal composition were considered.7 Level I biorelevant media reflect luminal pH and buffer capacity whereas Level II biorelevant media take additionally into account bile acid content, digestion products, and osmolality of luminal contents.7
In vitro models were evaluated by using two highly dosed APIs, sulfasalazine and L-870,810, formulated as immediate release products, and one colon targeting product, Asacol®, for which the coating polymer dissolves only at pH values greater than 7, and, therefore, release of the API during residence in the upper GI lumen is unlikely.
Sulfasalazine (MW 398.4) has a log P of 3.8811 but it behaves as a monoprotic ionized acid in the entire intestine [pKa1: 2.70 (acidic); pKa2: 0.90 (basic)] (http://pubchem.ncbi.nlm.nih.gov/compound/5384001). Sulfasalazine is a low permeability API which, after oral administration, reaches the lower intestine where it is metabolized by gut bacteria to the active ingredient mesalamine (mesalazine, 5-aminosalicylic acid) and to sulfapyridine.11, 12 Biorelevant solubility and dissolution data collected in the present study suggest that dissolution of 500 mg immediate release tablets is incomplete by arrival to distal ileum.
L-870,810 is the monosodium salt of [5-(1, 1-dioxothiazinan-2-yl)-N-[(4-fluorophenyl) methyl]-8-hydroxy-1, 6-naphthyridine-7-carboxamide] (http://pubchem.ncbi.nlm.nih.gov/compound/457930). The free acid form of L-870,810 (MW 430.5) has log P 2.09 and is partly ionized in the lower intestine (pKa 7.3).13 After oral administration of 400 mg free acid equivalent granules in the fasted state, absorption is limited by luminal dissolution and solubility.13
Asacol® (400 mg/tab) has a Eudragit® S coating that dissolves at pH ≥ 7.0 and mesalamine as the active ingredient.14 Mesalamine (MW 153.14) is ionized in the lower intestine (pKa1 = 2.30 and pKa2 = 5.69).15 It is a low permeability API16, 17 with log P value between −1.5 and 0.98.16, 18, 19 Mesalamine is a locally acting API used for the treatment of inflammatory bowel diseases and is often formulated as a product which ensures high local drug concentrations at the region of inflammation in ulcerative colitis and in Crohn's ileo-colitis.
Data of the immediate release products were compared with data collected under conditions simulating the environment in the upper intestinal lumen. Biorelevance of in vitro data was evaluated using existing in vivo data in humans, and, where possible, in silico modeling techniques.
EXPERIMENTAL
Materials
Sulfasalazine powder (98.1% purity) was from Fluka Analytical (Buchs, Switzerland), and Azulfidine® immediate release tablets (500 mg/tab; Pfizer, Berlin, Germany) were purchased locally.
L-870,810 anhydrous crystalline powder (99.9% purity), granules of L-870,810 containing 52.5% (w/w) L-870,810 (50%, w/w free acid), and hard gelatine capsules (for testing the granules) were provided by Merck Research Laboratories (Merck& Co., Inc., Kenilworth, NJ, USA). L-870,810 exhibits good chemical stability in the solid state. Granules of L-870,810 were comprised of standard non-functional excipients (standard sugars as fillers along with disintegrants, lubricants, and binders) with no surfactant incorporated. None of these excipients would be expected to affect drug solubility. Protection from light is necessary for long-term storage of L-870,810 (Merck data on file). Solutions were protected from light and stability of the API was confirmed for all procedures applied in the present investigation.
Mesalamine powder (≥99% purity) was from Sigma–Aldrich (Saint Louis, Missouri) and Asacol® tablets (400 mg/tab; Tillots Pharma AG, Switzerland) were purchased locally.
Egg phosphatidylcholine (Lipoid E PC® 99.1% purity, lot# 105019-1/14) was kindly donated by Lipoid GmbH (Ludwigshafen, Germany). SIF® Powder Original was kindly donated by biorelevant.com (Surrey, United Kingdom). All other reagents were of analytical or HPLC grade and purchased commercially.
Evaluation of Sulfasalazine Solubility in Intestinal Lumen and of Azulfidine® Tablets Dissolution in Upper Intestinal Lumen
Solubility Measurements in Media Simulating the Intestinal Environment
Equilibrium solubility of sulfasalazine was measured in Level I and II SIFileum, FaSSCoF, and FeSSCoF.7 Measurements were made with the shake-flask method at 37°C in triplicate. Adequacy of filtration to separate dissolved from undissolved material, and adsorption of sulfasalazine on to the filter were evaluated with preliminary experiments. Thirty milligrams of sulfasalazine were added in amber glass vials containing 3 mL of medium and shaken for 48 h. Samples were filtered through 0.45 μm regenerated cellulose filters (Titan® 2 HPLC Filter LT Brown; 17 mm; Sun SRi), after discarding the first 1 mL.
Dissolution Experiments Under Conditions Simulating the Environment in Upper Intestinal Lumen
Dissolution of Azulfidine® tablets in fluids simulating the environment in upper small intestine, that is, in FaSSIF-V2 and in FeSSIF-V2,20 were performed by using 250 mL of dissolution medium and the mini-paddle apparatus (Erweka DT6 model) with the paddle rotating at 75 rpm. Experiments were performed in triplicate at 37 ± 0.5 °C. Samples (2 mL) were filtered through 0.45 μm regenerated cellulose filters (Titan® 2 HPLC Filter LT Brown; 17 mm; Sun SRi), after discarding the first 1 mL. Upon collection of the filtrate and appropriate dilution with mobile phase, the sample was vortexed, and the API concentration was measured with HPLC-UV. The drawn sample volume was replaced with the same volume of blank dissolution medium, which was kept in a separate vessel at a temperature of 37 ± 0.5°C.
Two-Stage Single-Compartment Models for Evaluating Dissolution in Distal Ileum and Proximal Colon
Dissolution was studied in a single-compartment setup using a medium simulating the environment in the distal ileum (Stage 1, 0–2 h) and a medium simulating the environment in the ascending colon (Stage 2, 2–6 h). Although retention of non-disintegrating dosage forms at the ileocecal valve can occur, especially in the fasted state, residence of micropellets in distal ileum is generally shorter than 2 h.21, 22 In the present study, experiments lasted for 2 h to ensure adequate characterization of dissolution profile. Similarly, duration of experiments under conditions simulating the environment in the ascending colon was based on the average residence time in the region23 and the need for collecting adequate information for characterizing the dissolution process.
Change of composition of the medium simulating the conditions in the distal ileum (SIFileum) to a medium simulating the conditions in ascending colon in the fasted state (FaSSCoF) and in the fed state (FeSSCoF) was achieved by adding an appropriate solution at the end of Stage 1. The same medium simulating the conditions in the distal ileum (SIFileum) was used in both fasted and fed state, as recent data indicate that conditions at distal ileum are not affected significantly by dosing conditions.5 The ratio of volumes in Stage 1 and Stage 2 was 1:5, that is, similar to the average ratio of aqueous volumes in distal ileum and ascending colon of healthy adults.3, 5 Volumes used during Stage 1 and during Stage 2 were a compromise between the actual ratio of intraluminal volumes and practicalities, for example, ability to test various types of formulations and draw multiple samples (Tables 1 and 2). Compositions of SIFileum, of the added solutions, and of the resulting solutions (FaSSCoF and FeSSCoF) are presented in Table 1 (when fasted state conditions are to be simulated) and in Table 2 (when fed state conditions are to be simulated). Compositions were based on available literature data and assuming two levels of simulation of the luminal environment.7 In the first level of simulation (Level I), only the pH and buffer capacity was simulated. In Level II simulation, bile components, digestion products, and osmolality, are additionally simulated (Tables 1 and 2).
| SIFileumb | Added Solution | FaSSCoF | ||
|---|---|---|---|---|
| Sodium taurocholate (mM) | 0.8 | – | 0.15 | |
| Lecithin (mM) | 0.2 | 0.325 | 0.3 | |
| Sodium oleate (mM) | – | 0.125 | 0.1 | |
| Glucose (mg/mL) | – | – | – | |
| Tris (mM) | – | 45.4 | 45.4 | |
| Maleic acid (mM) | 52.8 | 75.8 | 75.8 | |
| NaOH (mM) | 105 | 120 | 120 | |
| Sodium chloride (mM) | 30.1 | – | – | |
| Osmolality (mOsm/kg) | 127 ± 0.6 (Level I) | 196 | ||
| 190 (Level II) | ||||
| Buffer capacity (HCl) [(mmol/L)/ΔpH)] | 10 | 16 | ||
| pH | 7.5 | 7.8 | 7.8 | |
| Volume (mL) | Mini-paddle apparatus | 40 | 160 | 200 |
| Flow-through apparatus | 50 | 200 | 250 | |
- a Level I simulation of composition in distal ileum (Level I SIFileum) and in fasted ascending colon (Level I FaSSCoF) result after eliminating data with bold characters. Actual volumes of solutions added to SIFileum to produce FaSSCoF in this study are also shown in the bottom of the table.
- b SIFileum was prepared using SIF® Powder Original.
| SIFileumb | Added Solution | FeSSCoF | ||
|---|---|---|---|---|
| Sodium taurocholate (mM) | 0.8 | 0.55 | 0.6 | |
| Lecithin (mM) | 0.2 | 0.575 | 0.5 | |
| Sodium oleate (mM) | – | 0.25 | 0.2 | |
| Glucose (mg/mL) | – | 17.5 | 14 | |
| Tris (mM) | – | 30.5 | 30.5 | |
| Maleic acid (mM) | 52.8 | 28.0 | 30.15 | |
| NaOH (mM) | 105 | 6 | 16.5 | |
| Sodium chloride (mM) | 30.1 | 26.63 | 34 | |
| Osmolality (mOsm/kg) | 127 ± 0.6 (Level I) | 67.7 ± 2.6 (Level I) | ||
| 190 (Level II) | 207 (Level II) | |||
| Buffer capacity (HCl) [(mmol/L)/ΔpH)] | 10 | 15 | ||
| pH | 7.5 | 5.65 | 6.0 | |
| Volume (mL) | Mini-paddle apparatus | 40 | 160 | 200 |
| Flow-through apparatus | 50 | 200 | 250 | |
- a Level I simulation of composition in distal ileum (Level I SIFileum) and in fed ascending colon (Level I FeSSCoF) result after eliminating data with bold characters. Actual volumes of solutions added to SIFileum to produce FeSSCoF in this study are also shown in the bottom of the table.
- b SIFileum was prepared using SIF® Powder Original.
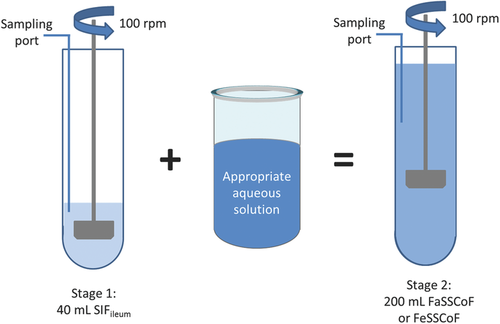
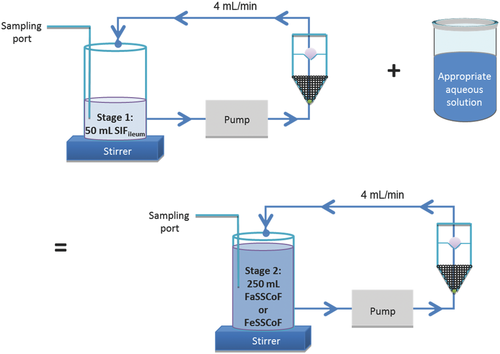
The impact of type/intensity of fluid convection was evaluated by using the mini-paddle apparatus with the paddle rotating at 100 rpm (Fig. 1) and the compendial flow-through apparatus (closed-loop mode) operating at 4 mL/min (Fig. 2).
To simulate dosing conditions previously applied in human studies13, 24, 25 and the presence of undissolved API in the lower intestine as much as possible, experiments were performed by using one Azulfidine® tablet under simulated fasted and fed state conditions (500 mg sulfasalazine), L-870,810 granules under simulated fasted state conditions [equivalent to 400 mg of API (free acid) placed in two hard gelatin capsules] and three Asacol® tablets under simulated fasted and fed state conditions (1200 mg mesalamine). It may be argued that after administration of three Asacol® tablets, transit through the upper GI lumen may vary among the three tablets. However, in the majority of cases, orally administered monolithic dosage forms accumulate at the ileocecal valve prior to colon arrival.26
Experiments with the mini-paddle apparatus were performed using a Distek dissolution apparatus in conjunction with a model 2100β dissolution system (Distek, New Brunswick, New Jersey). The lower end of the paddle during Stage 1 (volume of medium: 40 mL) was 1 cm from the bottom of the mini vessel and sampling was performed from the middle of the distance between the top of the paddle and the surface of the medium (Fig. 1). In order to achieve better mixing of the contents of mini vessel, during Stage 2 (volume of medium: 200 mL), the paddle was raised by 2 cm and sampling was performed from the middle of the distance between the top of the paddle and the surface of the medium (Fig. 1).
Experiments with the flow-through apparatus were performed with an Erweka flow-through dissolution apparatus (Erweka DFZ60, Heusenstamm, Germany) equipped with a piston pump (HKP60, Heusenstamm, Germany). A 5 mm-sized glass bead was placed in the tip of each dissolution cell and a total of 1.7 g of 1 mm-sized glass beads were added above the 5 mm glass bead. Just prior to initiation of an experiment, one Azulfidine® tablet was mounted on a holder whereas two L-870,810 capsules or three Asacol® tablets were put on top of the 1 mm-sized glass beads. On top of the cell, a combination of three glass fiber filters (MNGF-5, 0.4 μm pore size, 25 mm diameter; Macherey-Nagel, Germany), followed by 0.1 g glass wool and one more MNGF-5 glass fiber filter was used. Volume of dissolution medium was 50 mL during Stage 1 and 250 mL during Stage 2 (Fig. 2).
In all cases, experimental conditions and sample treatment were as described above for the evaluation of dissolution of Azulfidine® tablets in upper intestinal lumen. However, online filtration when using the flow through apparatus was not adequate for complete separation of solid particles, and additional filtration through 0.45 μm regenerated cellulose filters was necessary.
Analytical Methods
Sulfasalazine concentrations were measured with HPLC-UV. An Agilent Eclipse XDB-C8 column (4.6 × 150 mm2, 5 μm) was used. The mobile phase consisted of water:methanol (55:45, v/v). The flow rate was 0.4 mL/min. The detection wavelength was 365 nm. Injection volume was 20 μL. Elution time was approximately 5.5 min and limit of detection was 91 ng/mL. Slopes of calibration curves did not differ significantly from each other by accepting a Type I error of 0.05.
L-870,810 was assayed as described previously.13
Mesalamine concentrations were measured with HPLC-UV. A LiChrospher® 100 RP-18 endcapped, 5 μm, 250 × 4.6 mm2 column (Merck KGaA, Darmstadt, Germany) was used. The mobile phase consisted of phosphate buffer pH 7.4: methanol (95:5, v/v). The flow rate was 1.0 mL/min. The detection wavelength was 331 nm. Injection volume was 20 μL. Elution time was approximately 6 min and limit of detection was 182 ng/mL. Slopes of calibration curves did not differ significantly from each other by accepting a Type I error of 0.05.
Data Analysis
Immediate Release Dosage Forms
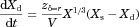 ()
()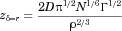 ()
()Data in FeSSCoF could not be collected (Azulfidine®) or were not collected (L-870,810 granules, because of the lack of human data in the fed state). As composition of SIFileum does not change with dosing conditions (Tables 1 and 2), only zδ = r values estimated from data collected under conditions simulating the fasted state were analyzed with analysis of variance.
Three factors were considered: the type/intensity of fluid convection (mini-paddle vs. flow-through, Factor A), the level of simulation of luminal composition in the lower intestine in the fasted state (Level I vs. Level II biorelevant media simulating fasted state conditions, Factor B), and regional differences in luminal composition of the lower intestine in the fasted state (distal ileum vs. ascending colon in the fasted state, Factor C).
In the case of Azulfidine®, zδ = r values from data collected with the mini-paddle apparatus in FaSSCoF could not be estimated reliably because of the rapid dissolution process. Thus, zδ = r values were analyzed with two, two-factor analyses of variance. Values estimated from data in SIFileum were analyzed for the impact of type/intensity of fluid convection (Factor A), and level of simulation of composition at distal ileum (Factor B). Values estimated from data collected with the flow-through apparatus were analyzed for the impact of level of simulation of composition at a specific region of the lower intestine in the fasted state (Factor B), and regional differences in luminal composition of the lower intestine in the fasted state (Factor C).
In the case of L-870,810 granules, zδ = r values were analyzed with three-factor analyses of variance (Factors A, B, and C).
In all cases, significance was evaluated accepting Type I error of 0.05. Tukey's post hoc test was applied, where needed. All relevant works were performed with Sigmastat (Ver.3.5; Systat Software Inc., San Jose, California).
Sulfasalazine data were qualitatively compared with previously collected sulfasalazine data in the plasma of healthy adults, after single dose administrations in the fasted and in the fed state.24
- It includes the zδ = r values that were estimated by the dissolution data collected under conditions simulating the environment in distal ileum and in ascending colon,
- It distinguishes intestinal residence into three different locations, upper small intestine (1.5 h), distal ileum (1 h) and ascending colon (3.5 h) instead of two locations assumed in the previous study (1.5 h in the upper intestine and 4.5 h in the lower intestine) and, thus, without affecting the total duration of absorption estimated previously from pharmacokinetic data,13 and
- It takes into account differences in solubility in distal ileum and ascending colon (estimated from the plateau levels of dissolution profiles, this study), and differences in the effective surface area in small intestine and ascending colon [based on differences in luminal fluid volumes (100 mL, small intestine/40 mL, colon) and radii (1.5 cm, small intestine/2 cm, colon)]. No magnification of the cylindrical area of the ascending colon was assumed, as it lacks folds and villi and being a highly permeable API, L-870,810 is considered to be absorbed from the tips of the microvilli.
Predicted plasma levels were evaluated versus individual actual plasma data from a previously conducted clinical study, after administration of 800 mg granules of L-870,810 (400 mg free acid) to healthy fasted adults.13
Asacol®
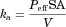 ()
()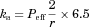 ()
()All estimations were performed using SigmaPlot v.11.0 software (Systat Software Inc.).
RESULTS
Evaluation of Sulfasalazine Solubility in Intestinal Lumen and of Azulfidine® Tablets Dissolution in Upper Intestinal Lumen
Sulfasalazine solubility data in Level I and II SIFileum, FaSSCoF, and FeSSCoF are given in Table 3. Level of simulation of composition in the lower intestine did not affect the data. Although a pH effect is apparent (solubilities in FeSSCoF are lower than in SIFileum or FaSSCoF), a solubilization effect in FaSSCoF could also be claimed [solubilities in SIFileum (pH 7.5) vs. solubilities in FaSSCoF (pH 7.8)], in line with previous data with other lipophilic APIs in FaSSCoF.6
| SIFileum | FaSSCoF | FeSSCoF | |
|---|---|---|---|
| Level I simulation of luminal conditions | 4.713 ± 0.073 | 8.18 ± 0.44 | 1.018 ± 0.047 |
| Level II simulation of luminal conditions | 3.94 ± 0.52 | 8.64 ± 0.11 | 0.994 ± 0.074 |
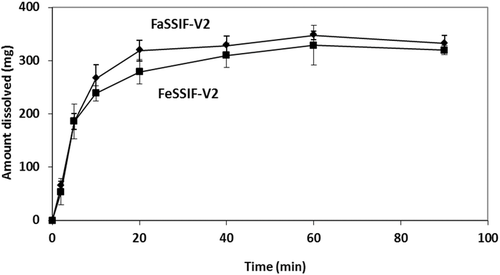
Estimated zδ = r values for the dissolution of Azulfidine® tablets in Level II FaSSIF-V2 and in Level II FeSSIF-V2 with the mini-paddle apparatus (Fig. 3) were 277 ± 33 mL mg−1/3 h−1, and 227 ± 145 mL mg−1/3 h−1, respectively. Based on the plateau levels observed in FaSSIF-V2 and in FeSSIF-V2 (Fig. 3), the ratio dose/solubility is more than 400 mL in these media and, therefore, it can be argued that dissolution of the dose in upper intestine is incomplete, at least in the fasted state.
Two-Stage Single-Compartment Models for Evaluating Dissolution of Azulfidine® Tablets in Distal Ileum and Proximal Colon
In SIFileum, the impact of type/intensity of fluid convection and the level of simulation of conditions in distal ileum on zδ = r values (Table 4) was not significant (p ≥ 0.275). Although the power of the test was low, even if the impact was significant it would have no practical importance because of the rapid completion of the process in all cases (Figs. 4 and 5).
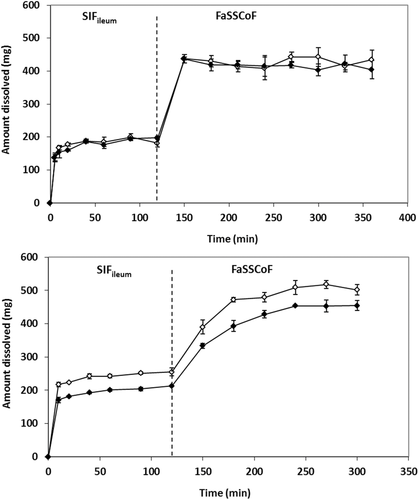
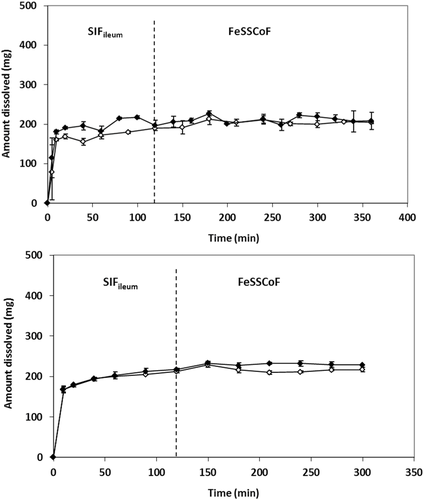
In FaSSCoF, dissolution data with the mini-paddle apparatus could not be discussed because of the lack of data points prior to the plateau level (Fig. 4). Data with the flow-through apparatus indicated that the level of simulation of luminal conditions affects zδ = r values (Table 4) significantly (p = 0.002), whereas the difference between data in SIFileum and FaSSCoF was also significant (p = 0.003).
Under fed state simulating conditions, the ratio [solubility in SIFileum/solubility in FeSSCoF] (Table 3) is 4.6 and 4.0 in Level I and Level II biorelevant media, respectively, that is, slightly smaller than the ratio [volume of FeSSCoF/volume of SIFileum] (5/1, Table 2) and no dissolution profile could be obtained in FeSSCoF (Fig. 5).
It is interesting to note that, using the mini-paddle apparatus, estimated mean zδ = r values in Level II SIFileum (Table 4) are about four times smaller than in Level II FaSSIF-V2 (69 vs. 277 mL mg−1/3 h−1) and more than three times smaller than in Level II FeSSIF-V2 (69 vs. 227 mL mg−1/3 h−1).
Two-Stage Single-Compartment Models for Evaluating Dissolution of L-870,810 Granules in Distal Ileum and Proximal Colon
In line with previous solubility data in human aspirates from the upper GI lumen in the fasted state and dissolution data in media simulating the conditions in upper GI lumen in the fasted state,13 dissolution of L-870,810 granules in SIFileum and in FaSSCoF was limited (Fig. 6).
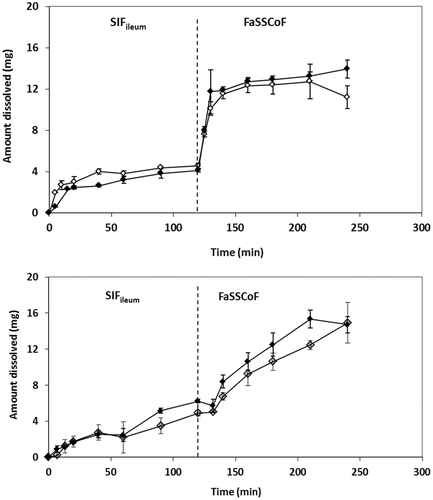
Compared with Azulfidine® tablets, dissolution of L-870,810 granules in SIFileum and in FaSSCoF was slower (Fig. 6 vs. Fig. 4). Three-way ANOVA confirmed that zδ = r values for L-870,810 granules (Table 5) are affected significantly by difference in composition between SIFileum and FaSSCoF, by the type/intensity of fluid convection, and by their interaction (p < 0.001 for all relevant comparisons). However, zδ = r values of L-870,810 granules are not affected significantly neither by the level of simulation of composition in lower intestine nor by the interaction of level of simulation with another factor. Therefore, differences in dissolution of L-870,810 granules in distal ileum and ascending colon are primarily due to pH differences with the effect of other luminal components being non-significant.
| Mini-Paddle Apparatus | Flow-Through Apparatus | |||
|---|---|---|---|---|
| SIFileum | FaSSCoF | SIFileum | FaSSCoF | |
| Level I simulation of luminal conditions | 60 ± 10 | Not availablea | 75.4 ± 3.7 | 130 ± 16 |
| Level II simulation of luminal conditions | 69 ± 20 | Not availablea | 66.9 ± 7.6 | 72 ± 12 |
- a Because of the lack of experimental data prior to the plateau level.
| Mini-Paddle Apparatus | Flow-Through Apparatus | |||
|---|---|---|---|---|
| SIFileum | FaSSCoF | SIFileum | FaSSCoF | |
| Level I simulation of luminal conditions | 23 ± 14 | 290 ± 66 | 10.0 ± 5.2 | 36.5 ± 3.5 |
| Level II simulation of luminal conditions | 9.9 ± 2.0 | 244 ± 73 | 9.1 ± 2.1 | 53 ± 15 |
Interestingly, using the mini-paddle apparatus, estimated mean zδ = r values in Level I SIFileum (Table 5) are more than five times smaller than in Level I FaSSIF-V2 (121 mL mg−1/3 h−1)13 and in Level II SIFileum (Table 5) are at about 38 times smaller than in Level II FaSSIF-V2 (377 mL mg−1/3 h−1).13 In contrast, estimated mean zδ = r value in Level I FaSSCoF (Table 5) is 2.4 times bigger than in Level I FaSSIF-V2 (121 mL mg−1/3 h−1)13 and in Level II FaSSCoF (Table 5) is slightly smaller than in Level II FaSSIF-V2 (377 mL mg−1/3 h−1).13
Two-Stage Single-Compartment Models for Evaluating Dissolution of Asacol® Tablets in Distal Ileum and Proximal Colon
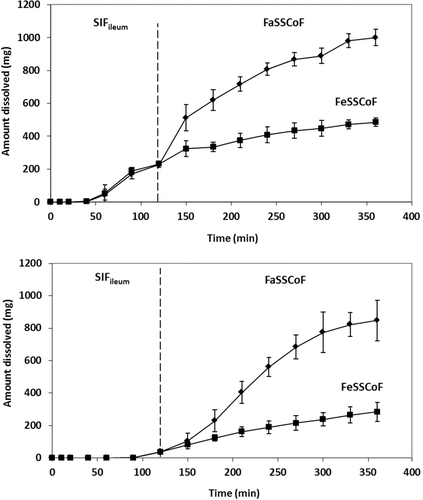
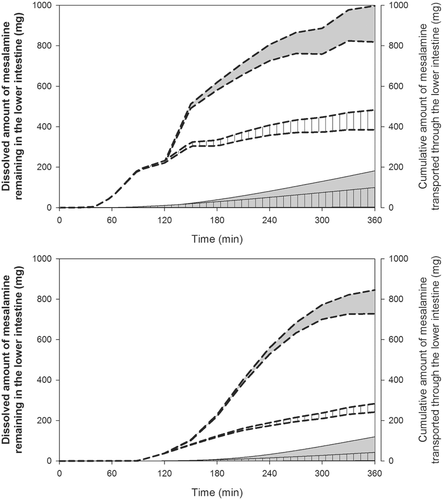
In line with data collected with the immediate release products with the two apparatus used in the present study, dissolution of Asacol® tablets using the mini-paddle apparatus was not affected by the level of simulation of luminal composition in the fasted state (data not shown). Consequently, experiments with Asacol® were completed with Level II simulated luminal fluids only. In SIFileum, amounts dissolved had low variability but they reached solubility level only with the mini-paddle apparatus (Fig. 7). The lower profiles obtained with the flow-through apparatus indicate an impact of type/intensity of SIFileum convection on dissolution kinetics. Data in FaSSCoF and FeSSCoF also indicate an impact of type/intensity of fluid convection; in addition, they indicate a negative food effect on dissolution of Asacol® tablets both with the mini-paddle and the flow-through apparatus (Fig. 7), primarily due to pH differences between FaSSCoF and FeSSCoF.
DISCUSSION
Dissolution data collected in the present study with immediate release formulations of two highly dosed APIs suggest that zδ = r values in distal ileum are smaller than in ascending colon (Tables 4 and 5) and in the upper intestine.13 In addition, dissolution is affected by the type/intensity of FaSSCoF convection but not SIFileum convection. In contrast, dissolution of Asacol® tablets was affected by the type/intensity of both FaSSCoF and SIFileum convection. These observations are in line with the previous data suggesting that dissolution becomes sensitive to hydrodynamics with the size of the dissolving solid.29 It is, therefore, interesting to evaluate further the importance of simulation of hydrodynamics in the lower intestine in predicting in vivo performance of colon targeted products, for example, by taking into account the low agitation intensity (estimated to be about 40 rpm, when using the paddle apparatus)30, the increased viscosity of contents in the distal ileum/ascending colon,5 and, perhaps, the shear stresses31 for evaluating potential dose dumping. In vitro data collected in the present study also suggest that the level of simulation of composition in the lower intestine does not have a substantial impact on the in vitro dissolution process. However, more APIs are needed to confirm this because contents of the ascending colon contain colloidal species,32 which can have substantial impact on drug solubility in the region,6 whereas the usefulness of bicarbonate buffer system in better predicting dosage form performance in the lower intestine, at least in the fasted state, may be worth investigating.
Data from the present study suggest that concentrations of sulfasalazine in the lumen of ascending colon should be lower under fed state conditions (Figs. 4 and 5). As sulfasalazine is not substantially absorbed from upper GI lumen,11 and in the lower intestine is degraded to sulfapyridine and 5-aminosalicylic acid, this observation should further indicate lower plasma levels of sulfasalazine and sulfapyridine, after single dose administration in the fed state. Indeed, after single dosage administrations of sulfasalazine to healthy adults in the fasted state and in the fed state (induced with a meal consisting of bread, egg, and orange juice) it has been observed that plasma Cmax and AUC values were lower in the fed state for both sulfasalazine and sulfapyridine.24
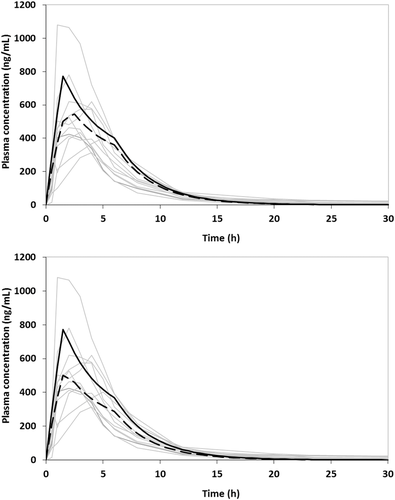
It has recently been reported that by simulating the luminal dissolution of L-870,810 granules using data in FaSSIF or FaSSIF-V2 and by estimating the effective surface area of intestinal mucosa assuming circular folds and villi magnifications during the entire absorption process, the average plasma profile, after single dose administration to fasted adults is grossly overestimated.13 By including the zδ = r values estimated in the present investigation for the lower intestine and by taking into account the solubility in and the effective surface area of the lower intestine, the average in vivo profile was adequately simulated (Fig. 8). It is important to note that the substantial effect of the level of luminal simulation7 on the plasma profiles is almost exclusively due difference in zδ = r values estimated in Level I FaSSIF-V2 versus Level II FaSSIF-V2 and not to the impact of level of simulation of environment in the lower intestine. For this particular example, differences in composition between SIFileum and FaSSCoF and type/intensity of convection did not have substantial impact on the declining phase of the profile, that is, immediately after Cmax.
Data from the present study suggest a negative food effect on luminal mesalamine concentrations from Asacol(R). However, nonsignificant food effect has been observed on mesalamine plasma levels, after single dose administrations of six Asacol® tablets (400 mg/tab) to healthy adults.25 It is important to note that administration of three Asacol® tablets (400 mg/tab) (tested in the present study) results in high concentrations of mesalamine in the lower intestine (greater than 1 mg/mL).28 Mesalamine transport through the mucosa of the lower intestine from such high luminal concentrations is predominantly paracellular and slow.16 Based on transport characteristics at high luminal concentrations, the cumulative amount of mesalamine transported through the mucosa of the lower intestine is limited by the transport through the mucosa rather than luminal concentrations (Fig. 9) and, therefore, no food effects on systemic availability, after administration of three or more Asacol® tablets (400 mg/tab) should be expected.
The two-stage single-compartment methodology proposed with the present study offers the possibility for evaluating dosage form performance in two locations of the lower intestine, distal ileum and ascending colon, with a single test. The physiologically relevant simulation of change in composition from distal ileum to the proximal colon may also be useful in assessing potential precipitation of API in the region. Such possibility is relevant for low solubility weak bases which are sensitive to the drop of the pH from the distal ileum into the cecum/ascending colon, especially in the fed state (please see pH values in Tables 1 and 2). In fact, some low solubility APIs have unexpectedly low degree of absorption in the colon despite being administered to the colon as solutions, implying in vivo precipitation during regional absorption studies.1 In vitro assessment of such precipitation would facilitate formulation selection prior to conduction of a regional absorption study and will help avoiding misinterpretation of data as it has been reported in previous regional absorption studies in dogs.33
One limitation of the proposed methodology is that estimation of dissolution characteristics of a highly dosed API is not possible in cases where solubility in medium simulating the conditions in the ascending colon is similar with or up to five times lower than in SIFileum. However, as the dilution ratio of aqueous portions of contents was based on luminal data, in those situations, dissolution is expected to be limited also in vivo, and to occur only in response to the elimination of dissolved material because of the transport via the colonic mucosa.
CONCLUDING REMARKS
In this investigation two-stage single-compartment models for evaluating dissolution in the lower intestine were proposed and a preliminary evaluation of their usefulness was performed.
Compared with multi-compartmental models or with separate experiments using single-compartment models, two-stage single-compartment models can facilitate experimentation whereas, in addition to dissolution, other processes such as potential precipitation during transfer from ileum to ascending colon may be possible to investigate. Proposed models are based on commercially available setups and they could, therefore, be considered for immediate application by industry and, perhaps, regulatory agencies.
Based on data collected using two highly dosed compounds and a colon targeting product, dissolution characteristics in the lower intestine can be much different from that in upper intestine with potential impact on PBPK modelling. In vitro data collected in this study were in line with previously collected in vivo data in healthy adults. However, the impact of type/intensity of fluid convection and viscosity of media on luminal performance of other APIs and drug products requires further exploration.
ACKNOWLEDGMENTS
Authors would like to thank Prof. W. Weitschies (Ernst Moritz Arndt University of Greifswald, Germany) for providing sulfasalazine and Azulfidine® tablets and Prof. J. Dressman (Goethe University, Frankfurt/Main, Germany) for providing mesalamine powder.
Part of the present work was presented as a poster at the “Progress within the IMI OrBiTo project – predictive tools for oral Biopharmaceutics” meeting (Academy of Pharmaceutical Sciences, Stevenage, UK, 13 May 2014).
This work has received support from the Innovative Medicines Initiative Joint Undertaking (http://www.imi.europa.eu) under Grant agreement no. 115369, resources of which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/2007-2013) and EFPIA companies' in kind contribution.




