Application of Physiologically Based Absorption Modeling for Amphetamine Salts Drug Products in Generic Drug Evaluation
Abstract
Amphetamine (AMP) salts-based extended-release (ER) drug products are widely used for the treatment of attention deficit hyperactivity disorder. We developed physiologically based absorption models for mixed AMP salts ER capsules and dextroamphetamine sulfate ER capsules to address specific questions raised during generic drug postmarketing surveillance and bioequivalence (BE) guidance development. The models were verified against several data sets. Virtual BE simulations were conducted to assess BE in various populations other than normal healthy subjects where BE studies are generally conducted for approval. The models were also used to predict pharmacokinetics (PK) for hypothetical formulations having dissolution profiles falling within specification after the development of in vitro–in vivo relation. Finally, we demonstrated how to use the models to test sensitivity of PK metrics to the changes in formulation variables. © 2015 Wiley Periodicals, Inc. and the American Pharmacists Association J Pharm Sci 104:3170–3182, 2015
INTRODUCTION
Physiologically based absorption modeling and simulation for oral dosage forms has demonstrated its value in various aspects of generic drug evaluation.1, 2 We present a case example where physiologically based absorption modeling and simulation was applied in bioequivalence (BE) guidance development and postmarketing surveillance risk assessment of generic drugs.
Over the last 10 years, the generics share of prescriptions has increased from 54% in 2003 to 84% in 2012 and will likely rise to 87% by 2017.3 Along with the efforts being devoted to developing and approving generic drugs is the establishment of a strong post marketing surveillance program for generic drugs. The Office of Generic Drugs (OGD) regularly evaluates the adverse events reported for generic drug substitution and analyzes the potential root cause(s). There are many aspects involved in analyzing postmarketing safety signals and searching for the potential root causes, such as drug product quality evaluation, re-evaluation of BE criteria and BE data, and identifying gaps between BE studies and pharmaceutical equivalence data supporting approval and the clinical use of the product. Physiologically based absorption models are valuable tools to identify the potential risks to successful generic substitution because these mechanism-based models integrate drug substance properties, physiological properties, and drug release mechanisms (i.e., formulation properties). For example, virtual BE simulations can be performed based on validated physiologically based absorption models in various patient populations that have not been tested in BE studies. The OGD also routinely develops BE guidance for specific drug products.4 Physiologically based absorption modeling has also been used to address various questions in guidance development.5 In the example in this report, physiologically based absorption modeling was used to evaluate the sensitivity of a series of BE metrics to a change in formulation parameters.
Amphetamine (AMP) drug substance refers to the racemic 1:1 mixture of two enantiomers with central nervous system stimulant activity: dextroamphetamine [d-AMP; S-AMP (IUPAC)] and levoamphetamine [l-AMP; R-AMP (IUPAC)]. Mixed AMP salts (MAS) products are nonracemic combinations of four salts in a 1:1:1:1 ratio (by weight): d-AMP sulfate, d-AMP saccharate, AMP aspartate, and AMP sulfate. The ratio of d-AMP salts to l-AMP salts in MAS products is 3:1, whereas the ratio of base d-AMP to base l-AMP is approximately 3.15:1. This combination of AMP salts was approved for the treatment of attention deficit hyperactivity disorder (ADHD). Adderall® (NDA 11522) is an immediate-release (IR) tablet formulation of MAS, and Adderall XR® (NDA 21303) is an oral formulation of MAS consisting of two types of pellets in the ratio of 1:1 (in terms of drug load) in a gelatin capsule: an IR pellet and a delayed-release (DR) pellet.6 The DR pellets are enteric coated and trigger the release in higher pH environment. The combination of these pellets is considered to be an extended-release (ER) product. Dexedrine® (d-AMP sulfate) ER capsules (NDA 17078), also known as Dexedrine Spansules®, are also indicated for the treatment of ADHD. Its active ingredient is one of the ingredients in MAS products. We conducted physiologically based absorption modeling and simulation for three drug products: two reference listed drugs (Adderall XR® and Dexedrine®), and a generic version of Adderall XR® (referred to as Product Y). The reason for the selection of Product Y for simulation is because it has a similar design as Adderall XR® with two types of pellets, except that the mechanism of the modified-release (MR) pellet is ER instead of DR.
METHODS
Modeling and Simulation Strategy
The developmental process of the model to predict d-AMP and l-AMP plasma concentrations is based upon the methodology described by Zhang et al.7 From a chronological perspective, the first models that were developed described d-AMP and l-AMP concentrations following administration of MAS formulations. The models describing pure d-AMP formulations were developed as modifications to the MAS models. Unless indicated, all input parameters/profiles and pharmacokinetic (PK) information were collected from multiple new drug applications (NDAs) and abbreviated NDAs (ANDAs).
The Model Drug Substances
All AMP salts are considered to be highly soluble drugs according to the criteria of the Biopharmaceutical Classification System8 (Table 1). The apical-to-basolateral (A–B) and basolateral-to-apical (B–A) permeability rates of d-AMP sulfate through Caco-2 at pH 7.4 was reported to be 79.7 × 10−6 and 33.9 × 10−6 cm/s, respectively.9 The values of these rates and a significant favoring of transport in the A–B direction are suggestive of complete gastrointestinal (GI) absorption, that is, AMP is a highly permeable drug substance. The metabolic pathway for AMP is not well-established; however, AMP is known to be a substrate of CYP2D6 that catalyzes its conversion to 4-hydroxy-AMP.10 The major metabolite is alpha-hydroxy-AMP, where the molecule and its derivatives account for 50% of the administered dose recovered in urine and AMP itself accounting for 30%–40% in urine. AMP has a half-life of 9.77–11 h (d-AMP) and 11.5–13.8 h (l-AMP). Intestinal metabolism of AMP as well as hepatic first-pass effect (FPE) has not been evidenced. It is assumed that FPE has minor impact on absolute bioavailability and as such, it was neglected in model building. Other physiochemical properties of d-AMP and l-AMP used in simulations are provided in Table 1.
| Formulation | Adderall® | Adderall XR® | Product Y | Dexedrine® |
|---|---|---|---|---|
| Dose | 30 mga | 30 mg | 30 mg | 15 mg |
| Parameters | ||||
| MW (g/mol) (AMP base) | 135.21 | 135.21 | 135.21 | 135.21 |
| log Pb | 1.7 | 1.7 | 1.7 | 1.7 |
| Solubility (mg/mL at pH 6.8) | 128.56b | 128.56b | 128.56b | 160.13c |
| pKad | 9.9 | 9.9 | 9.9 | 9.9 |
| Dosage forme | IR: Tablet | Mixed Multiple Doses - IR: Solution, DR: MultPart EntCoat | CR: Dispersed | Mixed Multiple Doses - IR: Solution, CR: Dispersed |
| Dose (mg) | 14.247 (d-AMP) 4.523 (l-AMP) | 7.1235, 7.1235 (d-AMP) 2.2615, 2.2615 (l-AMP) | 14.247 (d-AMP) 4.523 (l-AMP) | 11.008 (total d-AMP) |
| Dose volume (mL)b | 250 | 250 | 250 | 250 |
| Dissolution Model | Johnson | Z-Factor (Takano) | In Vitro Dissolution Profilef | Weibull Functionf |
| Particle density (g/mL)b | 1.2 | 1.2 | 1.2 | 1.2 |
| Mean particle radius (μm)b | 25 | 25 | 25 | 25 |
| Particle radius standard deviation (μm)b | 0 | 0 | 0 | 0 |
| Particle radius bin #b | 1 | 1 | 1 | 1 |
| Precipitation time (sec)b | 900 | 900 | 900 | 900 |
| Diffusion coefficient (cm2/s)b | 1.06 | 1.06 | 1.06 | 1.06 |
| fup (%)b | 69.17 | 69.17 | 69.17 | 69.17 |
| Rbpb | 1.24 | 1.24 | 1.24 | 1.24 |
| Permeability (cm/s)g | 2.3268 × 10−4 | 2.3268 × 10−4 | 2.3268 × 10−4 | 2.3268 × 10−4 |
- a Except for dose, all parameters are the same for the b.i.d. simulation.
- b Predicted by ADMET predictor, or default values in GastroPlusTM.
- c Predicted by GastroPlusTM from solubility versus pH data taken from ANDA reviews.
- d Reported in ANDA reviews.
- e Dosage forms selected in GastroPlusTM.
- f “CR: Dispersed” dissolution rate controlled by either inputted in vitro dissolution profile or Weibull function.
- g Optimized values.
The PK Model—MAS
No data from an intravenous dose of MAS is available. Rather, PK data after oral administration of 30 mg Adderall® IR tablets under fasting conditions were fit in the PKPlusTM module of GastroPlusTM with Hooke and Jeeves Pattern Search method with unity weighting of the objective function to determine the appropriate compartmental model for d-AMP and l-AMP as determined by optimal Akaike Information Criterion (AIC) values. The average subject weight of 79.28 kg (or 174.78 lbs) was inputted into the module for each isomer. With a 30-mg dose of Adderall®, it was calculated that in a single tablet, there is 14.247 mg d-AMP base and 4.523 mg l-AMP base.
The Absorption/Advanced Compartmental Absorption and Transit Model—MAS
Because the PK model parameters were derived from oral data, the volumes of distribution and systemic clearances (CL) were only apparent values, as the PK model did not account for the bioavailability of the Adderall® tablet dose. The conventional PK model from PKPlusTM was reoptimized in the context of the Advanced Compartmental Absorption and Transit (ACAT) model of GastroPlusTM along with an optimization of the effective human permeability (Peff), which was a parameter initially estimated from the ADMET Predictor module of GastroPlusTM (Table 2). All physiological parameters are provided in Tables S1–S3 in the Supporting Information. For the simulations, both paracellular transport model (suggested by GastroPlusTM) and bile salt effect were included in the model. The “Opt log D Model SA/V6.1” option was selected for absorption scale factor determination. MAS formulations contain four active pharmaceutical ingredients (APIs) that account for the two active enantiomeric moieties. Each AMP salt has different solubility properties and it is not possible to determine in the available PK data what portion can be attributed to each salt. In order to simplify this model, two drug records were produced in GastroPlusTM: one for d-AMP and one for l-AMP. It was assumed that the compound/API for that record was either a theoretical salt of d-AMP or l-AMP. Similar solubility values were observed with d-AMP sulfate and AMP sulfate, whereas d-AMP saccharate and AMP aspartate showed higher levels of solubility. As the predicted solubility and experimental solubility of the AMP salts were high, the impact of that parameter on the simulation was minimal. As such, the ADMET predictor values were chosen for the simulation, which were similar to the d-AMP sulfate and AMP sulfate values (Table 1). The applicability of this solubility assumption was evaluated with a parameter sensitivity analysis (PSA). The dosage form “IR: Tablet” was selected and the default human fasted physiology or human fed physiology was selected in GastroPlusTM with no deviations from default values.
| Parameters | Conventional PK Model | Adderall® | Adderall XR® | Product Y | Dexedrine® |
|---|---|---|---|---|---|
| d-AMP | |||||
| CL (L/(h•kg)) | 0.18346 | 0.1685 | 0.1907 | 0.1907 | 0.1907 |
| Vc (L/kg) | 3.2231 | 2.8876 | 2.6987 | 2.6987 | 2.6987 |
| T1/2 (h) | 12.18 | 11.88 | 9.81 | 9.81 | 9.81 |
| Absorption rate constant, Ka (1/h)a | 1.649 | N.A. | N.A. | N.A. | N.A. |
| Lag time (h)a | 0.467 | N.A. | N.A. | N.A. | N.A. |
| Peff (cm/s × 104)b | N.A. | 2.3268 | 2.3268 | 2.3268 | 2.3268 |
| Z-factor (mL/(mg•s)) | N.A. | N.A. | 1.878 × 10−6 | N.A. | N.A. |
| Tlag (h) | N.A. | N.A. | N.A. | N.A. | 1.84 |
| A (h^b) | N.A. | N.A. | N.A. | N.A. | 9.71 |
| b | N.A. | N.A. | N.A. | N.A. | 1.96 |
| SITT (h) | N.A. | N.A. | 5.29 | 5.29 | 5.29 |
| STT (h) | N.A. | N.A. | 0.90 | 0.90 | 0.54 |
| l-AMP | |||||
| CL (L/(h•kg)) | 0.16835 | 0.15511 | 0.1835 | 0.1835 | |
| Vc (L/kg) | 3.6219 | 3.2514 | 2.9957 | 2.9957 | |
| T1/2 (h) | 14.91 | 14.53 | 11.31 | 11.31 | |
| Absorption rate constant, Ka (1/h) | 1.606 | N.A. | N.A. | N.A. | |
| Lag time (h) | 0.463 | N.A. | N.A. | N.A. | |
| Peff (cm/s × 104) | N.A. | 2.3268 | 2.3268 | 2.3268 | |
| Z-factor (mL/(mg•s)) | N.A. | N.A. | 2.500 × 10−6 | N.A. | |
| SITT (h) | N.A. | N.A. | 5.29 | 5.29 | |
| STT (h) | N.A. | N.A. | 0.90 | 0.90 | |
- a Ka and lag time were not used in ACAT modeling
- b Peff was kept constant between the two isomers.
- N.A., not applicable.
For b.i.d. dosing of Adderall® tablets every 4 h (q4h), “Mixed Multiple Doses” was selected as the dosage form where a single dose is given at the start of the simulation and an additional dose is given 4 h into the simulation. In one simulation (Simulation 1), the switch to the human fed physiology model was the only modification made; and in the other simulation (Simulation 2), to account for the different population, the one-compartmental PK parameters were reoptimized.
Adderall XR® (MAS) ER capsules contain a blend of IR and DR pellets with a ratio of 1:1 in terms of drug load and exhibits a multiphasic release profile.6 The IR pellets begin to dissolve immediately after administration in the stomach, whereas the DR pellets only begin to dissolve following the disintegration of the enteric coat once the individual pellet has transited from the stomach. As the DR portion does not transit through the stomach as a monolith, enteric-coated pellets behave more similarly to ER formulations because the pellets continuously trickle out of the stomach into the duodenum.11 For pelleted enteric formulations in GastroPlusTM, the “DR:MultiPart EntCoat” dosage form is the most appropriate as it treats the transit of each pellet from the stomach to the small intestine individually and drug is released from the pellet only once it has entered the small intestine using the dissolution model set in GastroPlusTM. We directly mimicked the Adderall XR® formulation with a “Mixed Multiple Doses” dosage form with equal dosings of “IR: Solution” and “DR: MultiPart EntCoat.” The IR pellets were described by “IR: Solution” instead of “IR: Capsule” or “IR: Tablet” because: (1) all three IR formulations can be expected to behave similarly because of the high solubility and high predicted permeability of AMP; and (2) only a single dissolution model can be specified in a GastroPlusTM record and the dissolution model necessary for the DR portion could not be used to describe IR release rates. The dissolution from the DR pellets after exiting the stomach was described with the Z-factor (Takano) model,12 where dissolution rate was controlled via optimization of the Z-factor parameter (Table 2). Previous optimization runs poorly underestimated the Tmax of all ER formulations. In rat studies, it was discovered that acute administration of AMP inhibited intestinal transit, whereas chronic administration had no effect; both acute and chronic administration inhibited gastric emptying.13 Extrapolating these conclusions to human studies would imply that single-dose BE studies would observe subjects with delayed transit times in the stomach and small intestine. Further improvements to the Adderall XR® model were made by optimizing the stomach transit time (STT) and small intestine transit time (SITT) as well as a reoptimization of the PK parameters as a different population sample was used (Table 2).
Product Y ER capsules contain a blend of IR and ER pellets with a ratio of 1:1 in terms of drug load. In the development of the Product Y model, data from extensive comparative in vitro dissolution testing of Product Y against Adderall XR® served as input to the “CR: Dispersed” dosage form in GastroPlusTM. The ACAT parameters are kept the same as the Adderall XR® model (Tables 1 and 2). The one-compartmental PK model was also left the same because the Product Y PK data used is from the same crossover BE study where the Adderall XR® PK data were derived from to develop the Adderall XR® model. The simulated profiles from each testing condition were compared against the observed PK profile of Product Y using the coefficient of determination, R2, as a goodness-of-fit measure. Although most profiles were similar, the one using the approved dissolution method for Product Y had the largest value of R2 (0.985) and was selected for the model.
Model Modifications for d-AMP Sulfate
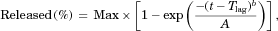 (1)
(1)Parameter Sensitivity Analysis
In the assessment of model parameter assumptions, PSAs were conducted on parameters that were predicted and arbitrarily assigned by varying one parameter at a time in a realistic span, whereas all other model parameters were kept constant. The relevant PK metrics resulting from simulation of each parameter value were collected. PSA results were evaluated by comparing the value of each metric associated with a parameter value to the value of the corresponding metric in the baseline simulation. Various other physiological and formulation-based parameters were also used in PSAs to identify theoretical populations and alternative formulations, respectively.
Simulation of PK After Administration of MAS ER Capsules in Various Subpopulations
Adderall XR® is approved for use in children aged 6 years and older.10 Starting with a healthy adult population, we used the fasting human physiology in GastroPlusTM optimized for MAS ER capsule products. The physiological parameters, including pH, permeability, transit time, and GI capacity were changed by referencing literature, as needed, to reflect the target subpopulations of pediatrics (ages 6–12) and adolescents (ages 13–17) (Bai et al., unpublished results). If the literature-reported adult value differed from the GastroPlusTM physiology, the GastroPlusTM value was kept, and pediatric and adolescent values were adjusted to keep the ratio to the adult value consistent. The elimination half-life, T1/2, is labeled as parameter that differs in adult, adolescent, and pediatric populations.10 For the pediatric population, the compartmental PK model was optimized to reflect the reported pediatric values of Cmax and AUC0–∞ while holding the reported pediatric T1/2 value constant. As adolescent values of Cmax and AUC0–∞ were not available, the adult PK model was used for the adolescent population. For Adderall XR® and Product Y, the physiology parameters and PK model were adjusted to reflect each population and the resultant mean profiles were directly compared.
Virtual BE Studies
In virtual BE studies between test and reference, PK profiles for 2000 different subjects were generated with 1000 subjects each for both formulations. Parameters for each virtual subject were randomly generated from log-normal distributions (Table S4). The coefficient of variation (CV) for each parameter is interpreted as intrasubject variability because of random selection of test and reference PK profiles. Because intrasubject variance for those physiological parameters are rarely available, CVs were arbitrarily constrained to 10% for the majority of the parameters so that the BE studies comparing reference versus reference can reach enough power with a moderate number of subjects for Cmax and AUC. This is consistent with the estimation of CVs from in-house two-way crossover study data. For each subject, the relevant PK metrics [Cmax, AUCt, and partial AUCs (pAUCs)] were calculated. For virtual trials, a specified number of randomly selected subjects (n = 12, 24, 36, 48, or 72) were sampled from each formulation (for n × 2 total subjects). Because the CVs were interpreted as intrasubject variability, our virtual BE simulations were interpreted as crossover studies, and average BE assessment was applied to analyze each relevant PK metric. If all the BE parameters passed the 90% confidence interval (CI) BE criteria (CI falls between 80% and 125%), the trial was classified as “pass”; otherwise, it was a “fail.” The trial was conducted 1000 times in total and the passing ratio was calculated and reported.
RESULTS
ACAT Model Simulations of AMP-Based IR and ER Formulations Closely Predicted the Observed PK Profiles After Oral Administration
One-, two-, and three-compartmental models were fitted to the observed plasma concentration–time profiles of both d-AMP and l-AMP from oral administration of 30-mg Adderall® tablets under fasting conditions to obtain the best-fit PK parameters in the PKPlusTM module of GastroPlusTM. As described in the Methods section, the four salts that make up MAS formulations were simplified to two theoretical salts, d-AMP salt and l-AMP salt, each with an assumed solubility. The one-compartment model was indicated as the best fit for each isomer. In addition, an improvement was observed when the model included lag time for oral dose. The apparent PK parameters described by these conventional PK models, systemic CL and central compartment volume (Vc), served as initial value estimates for the fitting of ACAT models to the same data sets along with a co-optimization of Peff. Optimization led to less than approximately 10% deviation for each parameter (Table 2). Both the conventional and ACAT models overpredicted the reported ranges of half-life in the Adderall® product label, but with slight improvement in the ACAT model. The simulated mean PK profiles for both d-AMP and l-AMP gave an accurate prediction of the observed mean PK profiles under fasting conditions using the optimized ACAT and PK parameters in Table 2 (Fig. 1a). The Cmax prediction differed from the observed mean value by approximately 4% for both isomers, whereas both AUC0–t and AUC0–∞ values were within 1% of observed (Table 3). For d-AMP, there was a slight overprediction of Tmax, whereas the l-AMP simulation gave the same Tmax as the observed data.
In the development of the ACAT model, because of the lack of information regarding certain physiochemical and PK properties of AMP and the formulation and compositional details of Adderall IR tablets, several parameters were unknown and were consequently assigned arbitrary values or were estimated by the ADMET predictor module of GastroPlusTM. Seven such parameters are: mean precipitation time, diffusion coefficient, reference solubility, particle density, mean particle radius, fraction unbound in plasma (fup), and blood-to-plasma concentration ratio (Rbp). Solubility was assigned a theoretical value reflective of the AMP salts with the lowest values. In addition, Peff was initially predicted off of ADMET predictor and subsequently optimized during model fitting. fup was predicted to be 69.17% by ADMET predictor; however, this differed from published value of about 20%.15 These eight parameters were varied in a PSA to assess model performance (see Figs. S1 and S2 in the Supporting Information). d-AMP and l-AMP models had nearly identical PSA results. Precipitation time, reference solubility, particle density, particle radius, fup, and Rbp had virtually no effect on any of the PK parameters. The result for fup demonstrated that the use of the predicted or experimental value has no impact on the AMP model. Peff had significant impacts on Cmax, AUC0–t, and Tmax as expected because lowering permeability delays the absorption of AMP into the bloodstream and increasing permeability leads to quicker absorption. The diffusion coefficient was observed to impact Cmax where decreasing values led to faster rate of absorption. It is important to note that the lack of impact on the PK parameters in the 100-fold range examined with the reference solubility parameter supports the assumption of using the software-predicted values and that the MAS product can be simulated as two theoretical d-AMP and l-AMP salts.
A simplistic confirmation of the developed AMP ACAT model was performed by observing how well the model could predict b.i.d. q4h dosing of Adderall® tablets, a dosing regimen comparatively assessed against Adderall XR® in Adderall XR®'s approval.6 Reoptimization of the PK model parameters showed improvement in the prediction (Fig. 1b). For d-AMP, Simulation 1 (dashed blue line) overpredicted Cmax and d-AMP is being cleared at too slow of a rate, whereas Simulation 2 (dashed red line) demonstrated improvement in prediction of the peak because of the first dose and a better prediction after 10 h. However, Simulation 2 still overpredicted Cmax by approximately 10% (Table 3). For l-AMP, Simulation 1 (solid blue line) predicted Cmax accurately while underpredicting the first peak and again the CL is lower than observed. Simulation 2 (solid red line) improved prediction of the first peak and the CL phase, but now Cmax is overestimated by approximately 10%. For Simulation 2, AUC0–t values were predicted accurately, whereas AUC0–∞ was 5% lower than observed for d-AMP and 10% lower for l-AMP. This deviation from the last time point of the simulation (24 h) to infinity can be explained by error in the optimized value of CL. Model improvement can be performed by optimizing the ACAT model to better reflect fed conditions; however, this is beyond the scope of this work. What we demonstrated here is that population variation between separate PK studies can be accounting for by tuning the PK model to match the behavior of the subjects in the individual study.
| Formulation | Adderall® | Adderall® | Adderall XR® | Product Y | Dexedrine® | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose | 30 mg | 10 mg b.i.d. | 30 mg | 30 mg | 15 mg | |||||
| Condition | Fasting | Fed | Fasting | Fasting | Fasting | |||||
| Parameters | Obs. | Pred. | Obs. | Pred.a | Obs. | Pred. | Obs. | Pred. | Obs. | Pred. |
| d-AMP | ||||||||||
| Cmax (ng/mL) | 50.17 | 47.977 | 26.225 | 29.139 | 45.09 | 45.758 | 45.01 | 45.724 | 24.029 | 24.717 |
| AUC0–t (ng•h/mL)b | 961.21 | 954.7 | 386.67 | 398.53 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| AUC0–∞ (ng•h/mL) | 988.31 | 990.77 | 507.91 | 481.86 | 902.48 | 878.03 | 896.19 | 865.59 | 698.87 | 641.97 |
| AUC0–x (ng•h/mL)c | N.A. | N.A. | N.A. | N.A. | 146.68 | 151.00 | 152.80 | 138.60 | 51.04 | 49.55 |
| AUCx–t (ng•h/mL)c | N.A. | N.A. | N.A. | N.A. | 732.04 | 709.28 | 718.02 | 709.07 | 577.47 | 531.57 |
| Tmax (h) | 2.5 | 2.8 | 7 | 7 | 5 | 4.8 | 5 | 5 | 8 | 7 |
| Fa (%) | N.A. | 92.884 | N.A. | 92.54 | N.A. | 92.743 | N.A. | 91.822 | N.A. | 73.383 |
| Correlation coefficient (R2) | 0.961 | 0.939 | 0.973 | 0.959 | 0.990 | |||||
| l-AMP | ||||||||||
| Cmax (ng/mL) | 14.42 | 13.807 | 8.543 | 9.4579 | 13.66 | 13.223 | 14.53 | 13.416 | ||
| AUC0–t (ng•h/mL) | 320.16 | 319.22 | 134.12 | 137.87 | N.A. | N.A. | N.A. | N.A. | ||
| AUC0–∞ (ng•h/mL) | 344.83 | 341.77 | 201.15 | 180.59 | 309.88 | 285.09 | 319.86 | 285.69 | ||
| AUC0–x (ng•h/mL)c | N.A. | N.A. | N.A. | N.A. | 42.29 | 37.97 | 46.60 | 40.17 | ||
| AUCx–t (ng•h/mL)c | N.A. | N.A. | N.A. | N.A. | 251.34 | 237.26 | 257.43 | 235.76 | ||
| Tmax (h) | 3 | 3 | 7 | 7.256 | 5 | 5.3 | 5 | 5.1 | ||
| Fa (%) | N.A. | 92.916 | N.A. | 92.52 | N.A. | 91.689 | N.A. | 91.885 | ||
| Correlation coefficient (R2) | 0.962 | 0.94 | 0.952 | 0.952 | ||||||
- a This is Simulation 2 of Figure 1b.
- b AUC0–t is not an evaluated metric for BE studies in which partial AUCs are calculated.
- c For MAS ER, x = 5 h; for d-AMP ER, x = 4 h.
- Percent deviations between predicted and observed are provided in Table S5 in the Supporting Information.
- N.A., not applicable.
Adderall XR® ER capsules, as a blend of IR and DR pellets, have a multiphasic release profile. This release pattern was incorporated in GastroPlusTM by modeling both pellet types independently. The close prediction of observed PK profiles by increasing both SITT and STT corroborated the extrapolation of effects on transit times from rat studies (Fig. 1c; Table 3). The pAUCs of AUC0–5 and AUC5–t were evaluated for this product as these are currently recommended in the BE guidance for MAS ER capsule generic products.16 For d-AMP, all predicted PK metrics were within 4% of the observed values. The l-AMP predictions were less accurate, particularly with AUC0–5 that had approximately 10% deviation from the observed value. This drop in accuracy could be expected because SITT and STT were optimized in the d-AMP simulation and carried over to the l-AMP simulation. The d-AMP model was given priority because: (1) it would be extended to d-AMP only products, and (2) given that there is approximately 3.15 times more d-AMP base in MAS formulations than l-AMP and that d-AMP is three to five times more potent than l-AMP in terms of blocking dopamine uptake,17 d-AMP is expected to have a larger impact on the efficacy of MAS formulations than l-AMP.
Product Y, a generic version of Adderall XR® ER capsules, was formulated using ER pellets instead of the DR pellets from the reference product. The in vitro dissolution profile using the approved dissolution method for Product Y describes the in vivo dissolution rate in the ACAT model, and as such simulated the release of the drug in vivo (Fig. 1d; Table 3). For d-AMP, all predicted PK metrics are within 5% of the observed, except for AUC0–5 that has approximately 10% deviation. Similar to Adderall XR results, there was a drop in model performance when predicting l-AMP concentrations. All l-AMP metrics, besides Tmax, deviate by 8%–14%. The Product Y model directly correlates in vitro dissolution to in vivo plasma concentrations; in other words, the model serves as an in vitro–in vivo relation (IVIVR).
After the creation of the MAS models, the ACAT and PK models developed for the ER formulations were extended to describe d-AMP plasma concentrations following administration of Dexedrine® ER capsules, a formulation that contains only d-AMP. The resultant PK profile was a fairly accurate fit to the observed Dexedrine ER capsule profile (Fig. 1e; Table 3). Consistent with BE guidance for d-AMP sulfate ER capsule generic products,18 the pAUCs of AUC0–4 and AUC4–t were evaluated for this product. The most significant departure between the observed and predicted profiles was in the underprediction of AUC0–∞ (deviation of ∼8%) in the Dexedrine® model. This could visually be observed in the CL phase. In the development of this model, the PK model was left the same as that used for Adderall XR® and Product Y. A reoptimization of the PK model could potentially lead to a better prediction of the CL phase, but the deviation was not as significant of a departure as observed in some of the other models.
Impact on BE of Different Release Mechanism Between Adderall XR® and Product Y in Various Subpopulations
ACAT models have been developed that predict the in vivo dissolution and in vivo absorption after administration of Adderall XR® and Product Y. These models account for the differences in the mechanism of release from both of these formulations. The DR pellets of Adderall XR® has pH-dependent release that is controlled by the “DR: MultPart EntCoat” dosage form in GastroPlusTM. The ER pellets of Product Y have pH-independent release and their release (along with the IR pellets) is controlled with an inputted in vitro dissolution profile. As a result, Adderall XR® and Product Y have distinctive predicted in vivo dissolution and in vivo absorption profiles (see Fig. S3 in the Supporting Information) that led to a conclusion of BE from the healthy adult population typical of BE studies. In this section, we altered parameters in GastroPlusTM to achieve population subsets where BE was re-evaluated for any potential issues. As d-AMP profiles and l-AMP profiles gave similar results, only d-AMP was extensively analyzed.
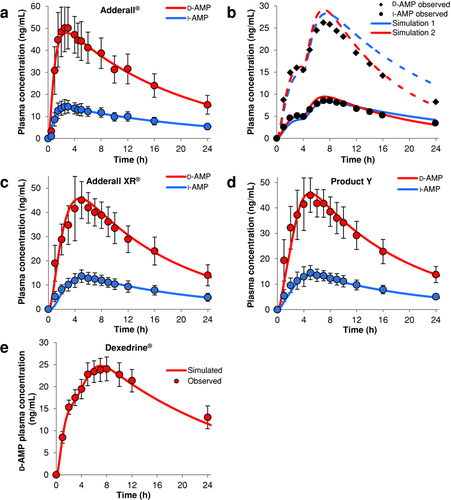
The first trial was conducted to discover whether variations in PK parameters across a population can affect the test-to-reference ratio [T/R; or point estimate (PE)] for various PK metrics, namely, Cmax, AUC0–∞, AUC0–5, and AUC5–t. Three PK model parameters were varied with a CV of 25%: CL, Vc, and subject weight. One thousand subjects were simulated, where the parameters above were randomly selected using the population simulation feature of GastroPlusTM. For each subject, the resultant PK metrics were plotted against T1/2, which was calculated from CL and Vc (Fig. 2a). Although the regression analysis demonstrated correlation between all PK metrics versus T1/2, the change in PE was practically negligible as the slopes were only slightly positive or negative. The models predicted that when only postabsorption changes were considered between individuals, both test and reference products continued showing BE (individual PEs between 0.8 and 1.25).
Changes in the baseline physiology/ACAT models for Adderall XR® and Product Y in GastroPlusTM can lead to changes in the in vivo absorption profile. The goal of the next trial was to determine physiological parameters that would be expected to differentially affect the two profiles (i.e., parameters that would demonstrate subject-by-formulation interaction). Considering a dosage form containing an enteric coat, a natural parameter to vary is the pH in the intestinal compartment in which the coating disintegrates or in the stomach. pH changes would be expected to have minor effects on the predicted Product Y PK profile. High gastric pH above the trigger pH of the enteric coating would essentially render Adderall XR® to be an IR formulation. Also, decreased pH in the initial intestinal compartments could lead to further delay of release from the DR pellets of Adderall XR®. However, the handling of DR dosage forms in GastroPlusTM does not allow a trigger pH to be set; instead, no drug is released in the stomach regardless of gastric pH, and all drug is released once it leaves the stomach regardless of the duodenal pH. Thus, with the current models, compartment pH cannot be further examined. Another critical parameter between the two formulations is how long the individual pellets reside in the stomach following ingestion of the capsules. Although in the stomach, the DR pellets of Adderall XR® do not dissolve and are not instantly available for absorption until they enter the duodenum, and the ER pellets of Product Y release drug throughout the time it resides in the stomach. To alter these conditions, a PSA was conducted with STT range from 0.05 to 2 h with logarithmic spacing for both products (Fig. 2b). In total, 501 different STTs were used, representing the same number of subjects. The baseline simulation (STT = 0.90 h) is included in Figure 2b as red diamonds. With increasing STT, we observed slight positive trends in the PEs for AUC0–∞, AUC5–t, and Cmax, indicating minimal risk of BE failure for these metrics. Larger deviations in PEs were observed with AUC0–5 as PEs approach 0.8 at lower STTs and near unity at higher STTs. For each product, AUC0–5 was observed to decrease with STT with the metric converging at highest STTs (see Fig. S4 in the Supporting Information). The low PE at low STTs is driven by higher AUC0–5 values for Adderall XR®, which is to be expected, because as mentioned, the product now behaves as complete IR formulation and more drug is immediately available for absorption. The value of 0.8 for the PE would indicate a risk to BE; however, the Product Y model underpredicted mean AUC0–5 (predicted mean PE 0.918; observed mean PE 1.042) and thus AUC0–5 PEs in Figure 2b are also underpredicted. Although STT has been shown to affect product performance, in the range of physiologically possible values for STT, there is minimal risk of BE failure, as we did not observe potential individuals outside BE limits.
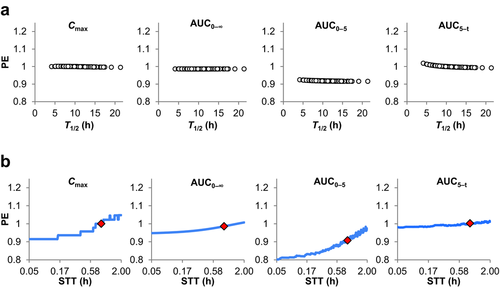
In the last two trials, we examined theoretical populations by varying certain parameters but keeping the rest of the physiologically parameters intact. We developed additional physiologies in GastroPlusTM to reflect pediatric, adolescent, geriatric populations as well as impacts on physiological parameters because of certain GI disease states. New parameter values were derived from published sources. Figure 3 reproduces the results of simulating Adderall XR® and Product Y in pediatrics, adolescents, and adults, as these are the populations most associated with ADHD medication. For pediatrics and adults, the extent and rate of absorption matched the values reported in the publically available Office of Clinical Pharmacology and Biopharmaceutics Review.6 For adolescents, no such data are available. As the adult PK model was used on a per-kg basis (e.g., CL divided by weight kept constant between adults and adolescents), differences in the representative subject body weights between adults and adolescents are the main source in difference between the two simulations and leads to higher observed plasma concentrations in the adolescent population. The PK profiles demonstrated minimal risk to BE when those additional populations are considered.
Use of Product Y IVIVR to Develop In Vitro Dissolution Specifications
Dissolution specifications for ANDAs have typically been for the purpose of product quality control and have often been set by variability observed in the batch used for the pivotal BE studies for approval. The simulated baseline profile of Product Y served as the reference profile for virtual BE trials. The dissolution profile used as input for the Product Y model is displayed in Figure 4 as “Reference.” The dissolution data show a plateau of around 50% for the first hour of the dissolution test (the IR portion). Any later dissolution is assumed to be from the ER pellets. This means that a specification at a later time of 55%–80% would correspond to an implicit specification of 10%–60% on the ER component at that time if we assume that the entire IR portion is dissolved by that time.
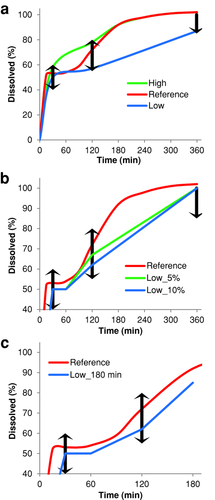
As the Product Y dissolution profile served as input for the ACAT model, it was possible to simulate the impact of the dissolution rates on BE to the biobatch (“Reference” profile) and evaluate different product specifications. We simulated different hypothetical dissolution profiles, namely, two profiles called “Low” and “High” that includes the lower bound and upper bound, respectively, of each criterion (Fig. 4a). It should be noted that the exact value of the lower bound and the upper bound were not used in the profiles. Instead, 2% above the lower bound and 2% below the upper bound were used as 2% was the typical variation observed in the dissolution data and would ensure that the majority of dissolution units would be within range. We simulated PK profiles using the theoretical dissolution profiles as input. For all profiles, we assumed that release from the IR portion remains consistent (i.e., 100% in 30 min) and we introduced variation through the ER component of the drug product. The “Low” profile exhibits the same plateau seen with the reference data, but has a slower dissolution rate from the ER pellets where the percent dissolved just passes the lower bounds of the acceptance criteria. The “High” profile exhibits rapid release (compared with reference) at early time points and converges with the reference profile at around 3 h. A virtual BE trial was conducted comparing each dissolution profile against itself, and “Low” and “High” against “Reference” (Table 4). The distribution of each parameter in the virtual trial is provided in Table S4 in the Supporting Information. The high passing ratios observed with the like-versus-like trials serve as internal control to verify that the population distributions are set at a level where a product is shown to be BE to itself, as expected. The “Reference” and “High” formulations can be considered to be BE, whereas “Reference” and “Low” are inequivalent. The mean PE between “Low” and “Reference” from 72 subjects repeated 1000 times is 0.771, 0.844, 0.815, and 0.845 for Cmax, AUC0–∞, AUC0–5, and AUC5–t, respectively, showing that the major point of failure in this trial is with Cmax and a batch exhibiting the “Low” profile would no longer be considered therapeutically equivalent to the original reference product.
| Number of | Reference Versus | Low Versus | High Versus | Reference | Reference | Reference Versus | Reference Versus | Reference Versus |
|---|---|---|---|---|---|---|---|---|
| Subjects | Reference | Low | High | Versus Low | Versus High | Low_10% | Low_5% | Low_180 min |
| 12 | 99.1 | 89.0 | 97.0 | 0.4 | 92.4 | 34.9 | 56.9 | 84.9 |
| 24 | 100 | 99.9 | 100 | 0.1 | 100 | 57.3 | 86.3 | 98.6 |
| 36 | 100 | 100 | 100 | 0.1 | 100 | 76.5 | 97.7 | 99.9 |
| 48 | 100 | 100 | 100 | 0 | 100 | 85.4 | 99.4 | 100 |
| 72 | 100 | 100 | 100 | 0 | 100 | 96.6 | 100 | 100 |
- Passing ratios are the number of trials that pass BE criteria all relevant PK metrics divided by the total number of trials.
As an extension to the dissolution profile analysis above, we conducted additional simulations using hypothetical in vitro profiles with slower release than “Reference” but faster than “Low” in order to determine limits on release that would ensure BE. At 120 min, the “Low” profile percent released is exactly 15% lower than “Reference.” We evaluated the impact of profiles that are 10% and 5% lower than “Reference” (i.e., 62% and 67% vs. 72%), which we call “Low_5%” and “Low_10%,” respectively, to reflect the impact when the allowed batch-to-batch variation is reduced (Fig. 4b). At 360 min, these new profiles were given 100% release. There was definite improvement in BE as the lower bound of the 120-min time point approaches “Reference” (Table 4). At 5% below reference, suitable passing numbers were achieved, but it is not plausible for such a tight limit to be set in an in vitro dissolution test. At 10% below reference, there was a definite improvement over the “Low” profile and with enough subjects, a BE study could be powered to pass BE criteria, but it is not given that “Low_10%” would be found BE in a normally sized study. There is a large difference in the percent dissolved between “Reference” and each variation of the “Low” profile. The “Reference” profile achieved at least 85% release in less than 180 min. To test the impact of this middle region, we simulated a new profile (called “Low_180 min”), in which we modified “Low” to have final point of 85% at 180 min instead of 100% at 360 min (Fig. 4c). Regardless of subject, “Low_180 min” is virtually BE to “Reference” (Table 4). These results demonstrated that the 120-min time point was not as critical to product performance as was the rate of drug release, which is better controlled by moving the 360-min specification to 180 min.
Impact of Formulation Parameters on Alternative pAUCs for Assessment of BE to Adderall XR® and Dexedrine ER®
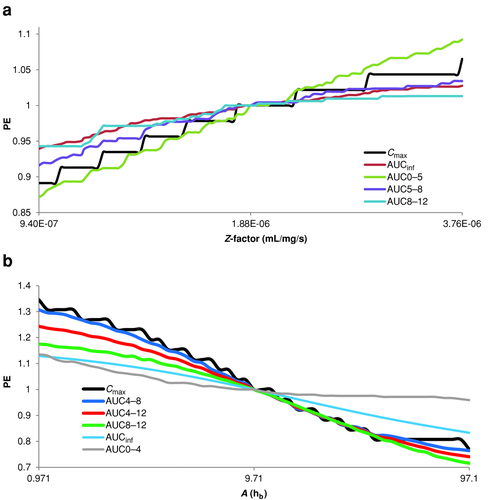
Adderall XR® was modeled as 50% IR and 50% DR, where the IR portion was treated as a solution and the dissolution rate of DR portion once leaving the stomach was controlled by a Z-factor (Takano) model. This same Adderall XR® model could also be used to model potential generics with similar release mechanism to Adderall XR®. To examine the range of alternative Adderall XR® formulations, we focused on only different dissolution rates from the DR portion where we also assumed that the 50:50 ratio between IR and DR components remains constant and the IR portion releases the same across all formulations. Different dissolution rates were achieved by performing a PSA on the Z-factor while evaluating several different pAUCs (Fig. 5a). AUC5–8 and AUC8–12 were included in this analysis because of the similarity in new pAUC metrics recommended for Concerta® (methylphenidate hydrochloride) ER tablets,19 a product with similar indication. Regardless of whether the DR portion has slower or faster release, both Cmax and AUC0–5 were predicted to have greater deviations in the PE from unity than the AUC5–8 and AUC8–12. We were unable to produce a theoretical formulation that passes the current recommended metrics but fails either AUC5–8 or AUC8–12 or both by only varying the Z-factor. In other words, if Cmax and AUC0–5 passed, then AUC5–8 and AUC8–12 passed as well. Cmax and AUC0–5 were the more sensitive measures to detect formulation differences in Adderall XR®-like products.
Similar to Adderall XR®, the Dexedrine® model is composed of a particular ratio of IR and MR pellets. We modified this ratio in the “Mixed Multiple Doses” definition in order to generate alternative formulations of Dexedrine®. A virtual BE study was conducted between these new formulations and Dexedrine® in order to determine the impact of IR–MR ratio on BE (Table 5). The baseline Dexedrine® ER model is referred to as “IR–MR.” The numbers 10 and 20 in “IR+10:MR-10” and “IR+20:MR-20” indicate the amount of percentage change for each component. Instead of the full range of population parameters in Table S4 in the Supporting Information, a smaller subset was used, which included only lognormal distributions of CL, Vc, STT, SITT, and Peff (Table S6 in the Supporting Information). In order to mimic the population sample from which the Dexedrine® ER capsules model was designed from, the CV of each parameter was adjusted (the same value was used for all parameters) until the CV of the Cmax of the simulated population matched the CV of the individual source data. The final CV determined for the parameters was 30%. We examined each PK metric independently rather than report an overall passing ratio. Traditional BE evaluations are indicated by Cmax and AUC0–∞. “IR+10:SR-10” would be able to pass both metrics; however, “IR+20:SR-20” would have issues passing Cmax. These results indicated that it would take a large deviation in IR–MR ratio before BE failure was achieved. AUC4–24 showed minor variation from the AUC0–∞ results and AUC4–12 (which contains Cmax) is less sensitive than Cmax. For AUC0–4, neither “IR+10:MR-10” nor “IR+20:MR-20” could pass BE criteria, particularly for “IR+20:MR-20” where in 5000 total trials, not a single one could produce a PE for the metric between 0.8 and 1.25. Those simulations suggested that AUC0–4 is a sensitive metric to detect differences because of variation in IR–MR component ratio.
| Trial | Number of Subjects | Cmax | AUC0–∞ | AUC0–4 | AUC4–12 | AUC4–24 |
|---|---|---|---|---|---|---|
| IR:MR Versus IR:MR | 12 | 62.0 | 45.4 | 71.9 | 65.7 | 69.6 |
| 24 | 96.3 | 90.0 | 97.9 | 96.5 | 97.5 | |
| 36 | 99.7 | 97.7 | 99.9 | 100 | 99.9 | |
| 48 | 100 | 99.7 | 100 | 100 | 100 | |
| 72 | 100 | 100 | 100 | 100 | 100 | |
| IR:MR Versus IR+10:MR-10 | 12 | 54.9 | 44.5 | 7.4 | 56.5 | 65.4 |
| 24 | 82.5 | 80.1 | 7.5 | 84.6 | 90.6 | |
| 36 | 94.3 | 94.1 | 8.0 | 95.8 | 98.7 | |
| 48 | 98.7 | 98.7 | 10.0 | 99.1 | 99.8 | |
| 72 | 99.8 | 99.8 | 9.3 | 99.9 | 100 | |
| IR:MR Versus IR+20:MR-20 | 12 | 23.2 | 34.9 | 0 | 25.2 | 43.4 |
| 24 | 39.6 | 61.2 | 0 | 44.7 | 69.0 | |
| 36 | 50.7 | 79.1 | 0 | 58.3 | 85.2 | |
| 48 | 61.0 | 84.9 | 0 | 67.0 | 90.6 | |
| 72 | 78.3 | 96.6 | 0 | 83.9 | 97.8 |
- Passing ratios are the number of trials that pass BE criteria all relevant PK metrics divided by the total number of trials.
For a second set of virtual trials conducted with Dexedrine®, a wide range of alternative formulations were investigated by modifying the Weibull function that controls the rate of release from the MR pellets while keeping the IR–MR ratio unchanged. A PSA was conducted using only the parameter A and multiple pAUCs were evaluated (Fig. 5b). Representative dissolution profiles (MR portion only) as described by the extreme values of A in the Weibull function and the resultant PK profiles can be found in Figure S5 in the Supporting Information. When considering time scales less than baseline (i.e., A = 9.71) that is indicative of formulations with faster MR release, a given formulation was more likely to fail Cmax before any of the AUCs, that is, all AUCs are less sensitive than Cmax. Interestingly, AUC0–4 was the least sensitive metric. This is indicative that changing MR release rates effect time points past 4 h and that early pAUCs are unable to detect such formulation differences. Although later pAUCs were able to detect and fail formulations, Cmax was even more sensitive. When considering time scales greater than baseline, the later pAUCs had about equal sensitivities as Cmax. At the highest time scales, the pAUCs surpassed Cmax in sensitivity; however, this was occurring in a region where PEs were out of BE limits. For the entire time-scale range, we observed that AUC4–8 tracked closely to Cmax, most likely because of the fact that Cmax was contained in that AUC interval.
DISCUSSION
Mechanistic oral absorption modeling and simulation has been used to address various questions involved in the context of generic drug evaluation.1, 2, 5, 7, 20 In this report, we conducted physiologically based absorption models for three AMP salts-based ER products: Adderall XR®, a generic version of Adderall XR® (Product Y), and Dexedrine®. After the models were confirmed against several data sets, they were then used to simulate BE studies to address specific questions, such as the impact of different release mechanisms between products on BE in various populations, impact of in vitro dissolution profiles on in vivo performance, and sensitivity of various PK metrics to the change of formulation differences.
It is notable that the model products are fairly complicated as multiple salts are included in a single formulation (MAS ER capsules) and they are ER formulations with multiphasic components. To develop the model for MAS ER capsules, we have created two hypothetical salts to account for d-AMP and l-AMP. Fortunately, the solubility of the AMP salts is high, although slightly different from each other, and it does not have much impact on PK profiles. To simulate the multiphasic components, “mixed multiple doses” files were created to contain IR and MR components, respectively.
It is also notable that in this report, we have applied the physiologically based absorption model to evaluate BE for various scenarios. We can evaluate whether higher risks of failure may be associated with generic products that are formulated in a different release mechanism from the reference product. For example, a pH-dependent release formulation and a pH-independent release formulation may perform differently when the GI luminal pH and GI transit time deviate from the mean physiology of a healthy subject. As explained in the “Results,” a higher gastric pH, such as subjects taking proton pump inhibitors, may trigger the release of an enteric-coated product so that it performs more like an IR product. This effect may not be expected for a pH-independent formulation. On the contrary, if a subject has a prolonged gastric empting time, the pH-independent formulation may start to release in the stomach while the release of the pH-dependent may be delayed because of the delayed emptying to the small intestine. Physiologically based absorption models provide quantitative insight to those theoretical analyses. On the basis of simulation, we identified that PEs of test-to-reference ratios of various PK parameters deviate from baseline for a subpopulation with prolonged STT (Fig. 2b). The risk of BE failure is considered minimal as no potential individuals were observed outside BE limits. The impact of gastric pH on BE was not tested as the trigger of the release of a DR dosage form is the location of the pellet (i.e., stomach or duodenum) rather than pH in GastroPlusTM. Nevertheless, physiologically based absorption modeling and simulation can be considered as a risk assessment tool from the aspect of analyzing potential failure of BE for formulations designed differently.
Generally, BE studies are conducted in healthy adult population. It is of great interest to evaluate BE in children and adolescents in this case as these drug products are widely prescribed in those populations. Physiologically based absorption models allow us to build virtual children and adolescent populations that have different GI physiology and PK parameters (CL, Vc, and body weight). Virtual BE trials were conducted in those populations. Simulations suggested that although bioavailability was different in those populations, the PE of test-to-reference ratios for PK metrics were close to those estimated in healthy adult population (Fig. 3).
In vitro–in vivo correlations (IVIVC) are expected for an ER drug product containing short half-life, high solubility, and high-permeability API. In this study, we tested various available dissolution profiles measured under different conditions as input profiles for Product Y and Dexedrine® ER capsules. We were able to identify a condition that simulated PK profile for Product Y but not for Dexedrine® ER capsules. Strictly speaking, to develop an IVIVC, the dissolution condition should be tested for several formulations to demonstrate that the in vitro difference is reflective of in vivo difference. In this report, the dissolution profile is predictive of the in vivo PK profile for Product Y. Whether the dissolution condition is able to predict the differences of in vivo performance is unknown, as only one formulation was tested. We refer this scenario as an IVIVR. Using the IVIVR, we simulated PK profiles using virtual dissolution profiles and conducted BE analysis comparing them with the biobatch (“Reference”). Simulation suggested that the 3-h time point, not the 6-h time point, is critical to ensure products within specification are BE to the “Reference” (Table 4).
Rather than drawing the conclusion of BE or bioinequivalence for a virtual formulation based on a single BE trial, we conducted thousands of BE trials using different number of subjects (Tables 4 and 5) and calculated the probability of passing BE criteria. By doing so, we could minimize the chances of drawing wrong conclusions based on a single BE trial. To incorporate intersubject and intrasubject variability, ideally the physiology parameters for a virtual subject should be obtained by the following steps: (1) sample an individual mean from the population mean and intersubject variance; (2) sample an individual intrasubject variance from an intrasubject variance distribution; and (3) sample the individual physiology parameters for treatment A (test), and treatment B (reference) based on the individual mean and intrasubject variance. There are several challenges to achieve those steps. First, the intrasubject variance is not well understood for each physiology parameter. Second, those steps were not integrated in GastroPlusTM. We simplified the procedure by omitting steps (1) and (2) so that all subjects have the same individual mean and intrasubject variance. Introducing the same individual mean may have minimum impact on the BE conclusions as those are crossover studies. However, variation in intrasubject variance may have an impact on the BE conclusions. In all the simulations, we used reference versus reference comparison as internal control to verify that the CVs of the virtual studies were consistent with those observed from in house BE studies.
Finally, we also demonstrated using the physiologically based absorption models to test the sensitivity of various PK metrics to the formulation change. The value will be greatly extended if the IVIVC is established and/or if there is a pharmacodynamic model linked to the PK model. With an IVIVC, physiologically based absorption and PK model, and an optional pharmacodynamic model, scientists can map a design space to inform drug development, and reduce the time and cost on trial and error for generic drug development. It is also of great value for regulatory scientists to develop appropriate BE criteria.
To summarize, in this study, we conducted physiologically based absorption modeling and simulation for MAS ER capsules and d-AMP sulfate ER capsules. The models were verified against several data sets. We further demonstrated several applications of the absorption models. Virtual BE simulations were conducted in various populations to address the BE concerns in different populations where BE studies are not conducted. Virtual BE simulations were also conducted for virtual formulations falling within the specification limits to identify potential risks associated with wide specification limits. The sensitivity of various PK metrics was also tested to the formulation change to evaluate the appropriateness of those PK metrics as BE metrics. During the course of this work, we also identified several areas to be improved in the physiologically based absorption modeling field, such as model improvement for the colon absorption for ER oral drug products, model improvement for multiple simultaneous dissolution models for complex multicomponent products, and better estimation of intrasubject variability for BE simulation.
ACKNOWLEDGMENTS
The authors would like to acknowledge the reviewing of modeling results by Drs. Lanyan Fang and Robert Lionberger, and the contribution of GI physiological parameters in various age groups from Dr. Jane Bai.
The authors declare no conflicts of interest. The views expressed in this article are those of the authors and do not necessarily reflect the official views of the US Food and Drug Administration.




